C3G and primary IC-MPGN are typically diagnosed in adolescence or early adulthood,2,6 but many patients report considerable diagnosis delays due to:
- Lack of disease awareness
- Heterogeneous clinical presentation, often overlapping with other glomerular diseases which can lead to misdiagnosis, delays and untreated renal damage1-3,9
- Histological overlap, requiring immunofluorescence to distinguish between C3G and IC-MPGN1,3
- Animal models suggest that C3 deposits may accumulate before clinical symptoms emerge, meaning irreversible damage may already be present at the time of diagnosis9-10
Low serum C3 levels is a common feature and may aid in the diagnosis. In many cases presentation is identical to acute post-infectious glomerulonephritis, however circulating C3 remains low for more than 8-12 weeks and over time disease does not resolve.2-3,7-8
>50% of children with C3G or primary IC-MPGN present with nephrotic syndrome and elevated serum creatinine.3,9
In some cases, children are diagnosed with low proteinuria/haematuria and low serum C3 levels in the absence of any clinical sign of the disease and with normal kidney function, during routine blood/urine tests.3
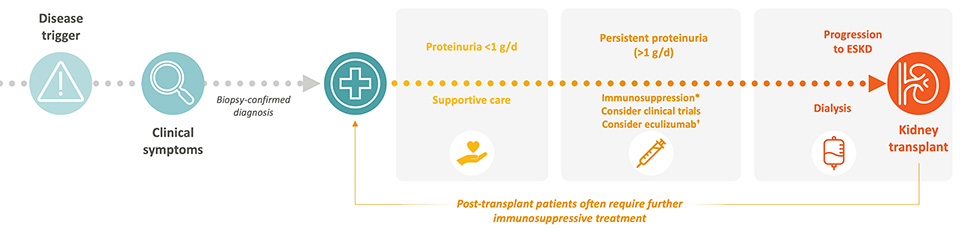
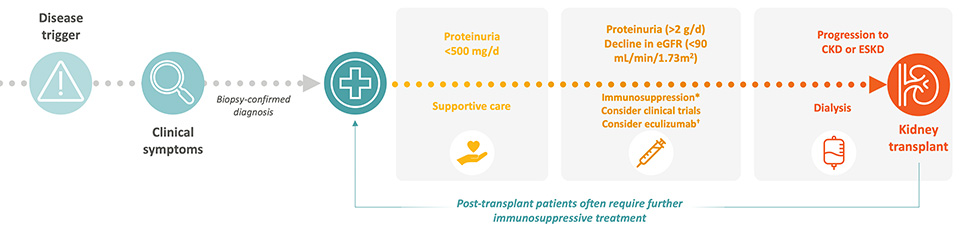
Supportive Therapy
ACE inhibitors and ARBs are recommended as first-line treatment options with the aim to prevent proteinuria. 3,7-9
While widely used, their benefit in C3G and IC-MPGN is extrapolated from other kidney diseases, and they do not address the underlying complement dysregulation.7,9
Immunosuppressive Therapy
In paediatric patients with moderate (over 0.5 g/g proteinuria) or severe disease (over 2 g/g proteinuria), and in adult patients with proteinuria > 1g/d, off-label use of MMF with or without corticosteroids is considered.3,8
The rationale is based on MMF’s immunomodulatory effects, but evidence is limited to retrospective studies with inconsistent outcomes.2-4,7-8
Complement Inhibition
Terminal complement inhibitors have been used off-label in a minority of patients with crescentic or rapidly progressive disease.2,8 However, clinical trials have not demonstrated efficacy of terminal complement inhibitors in C3G or primary IC-MPGN, likely due to its limited impact on upstream C3 activation.2-3,5,8,11
A proximal complement inhibitor that targets Factor B in the alternative pathway has been approved for use in adults with C3G only.12
Despite these treatment algorithms in C3G and primary IC-MPGN, patients continue to progress to ESKD.5
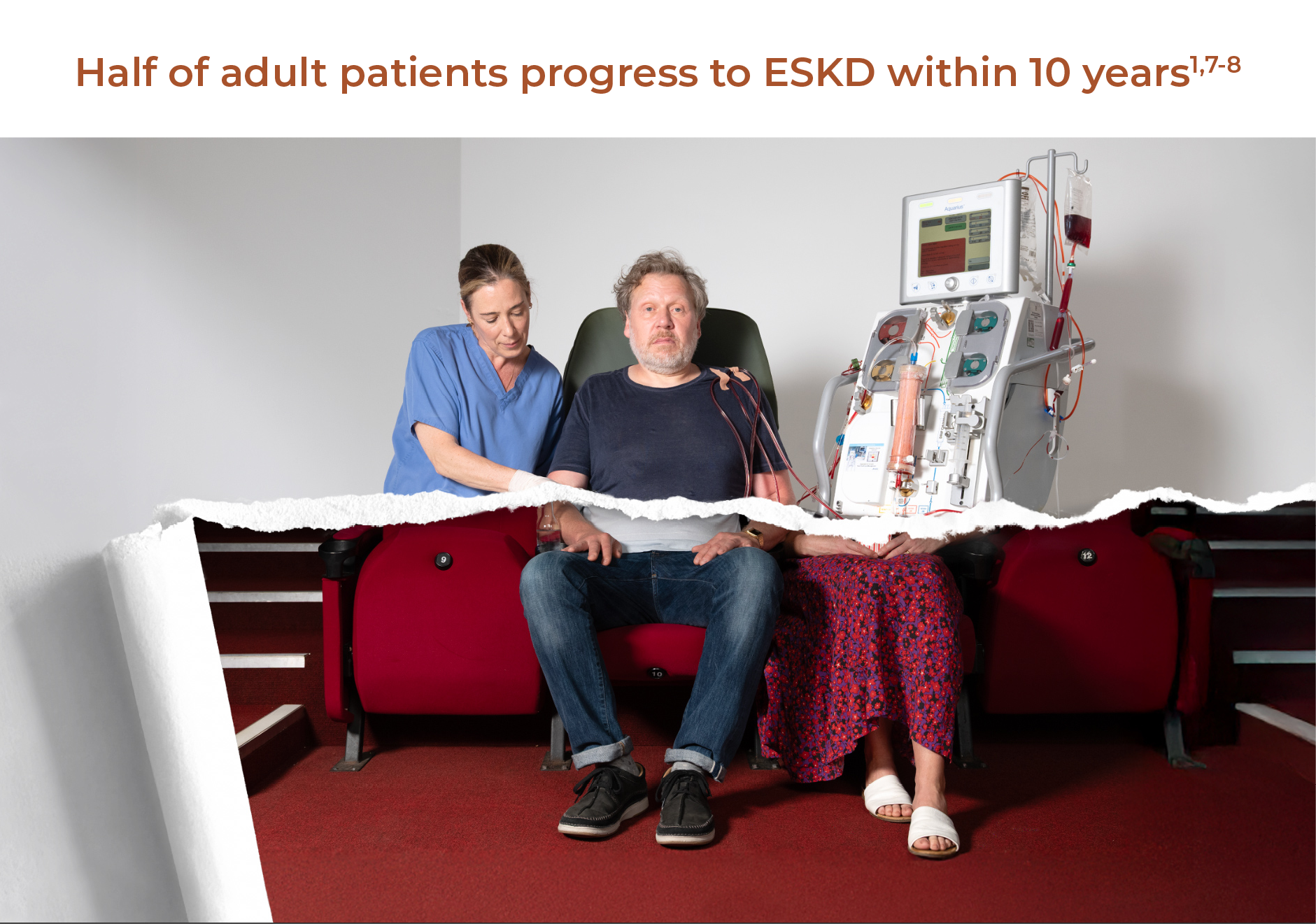
˜50% of adult patients progress to ESKD within 10 years1,7-8
Up to 80% of adult patients experience clinical disease recurrence after transplantation, with a 76% greater risk of mortality vs other nephropathies2,15
˜20% of paediatric patients reach ESKD within 10–15 years9
Up to 55% of paediatric patients experience clinical disease recurrence within 5 years post-transplant6
Dialysis is associated with reduced quality of life, increased hospitalisation, and a 25% mortality rate in the first year16
*Immunosuppressive therapies are associated with a high risk of adverse events, including serious infection, bone disease, dysglycaemia, obesity, hypertension, psychosis, gastrointestinal bleeding, cataracts and cardiovascular disease.4
†Anti-Ab and/or anti-CD19/20 therapies have been prescribed in severe cases but there is limited supporting evidence in the literature.2,11
>ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; C3, complement 3; C3G, C3 glomerulopathy; ESKD, end-stage kidney disease; IC-MPGN, immune complex-mediated membranoproliferative glomerulonephritis; KDIGO, Kidney Disease: Improving Global Outcomes; MMF, mycophenolate mofetil.
References:
- Cook HT & Pickering MC. Nat Rev Nephrol 2015;11:14–22.
- Caravaca-Fontán F, et al. Nephron 2020;144:272–80.
- Vivarelli M, et al. Pediatr Nephrol 2021;37:521–35.
- Jefferson JA. Clin J Am Soc Nephrol 2018;13:1264–75.
- Regunathan-Shenk R, et al. Am J Kidney Dis 2019;73:316–23.
- Patry C, et al. Pediatr Nephrol 2024; doi:10.1007/s00467-024-06476-5 [online ahead of print].
- Smith RJH, et al. Nat Rev Nephrol 2019;15:129–43.
- Heiderscheit AK, et al. Am J Med Genet C Semin Med Genet 2022;190C:344–57.
- Licht C, et al. Membranoproliferative glomerulonephritis and C3 glomerulopathy in children. In: Pediatric Nephrology. 2022. Springer Nature Switzerland, AG. pp 563–93.
- Jansen JH, et al. Am J Pathol 1993;143:1356–65.
- Michels MAHM, et al. Pediatr Nephrol 2022;37:601–12.
- Fabhalta 200mg hard capsules. Summary of Product Characteristics.
- Ayehu G, Atari M, Hassanein M, et al. C3 Glomerulopathy 2024. Available at: https://www.ncbi.nlm.nih.gov/books/NBK609090/. Accessed September 2025.
- Rovin BH, et al. Kidney Int 2021 Oct;100(4S):S1-S276.
- O'Shaughnessy MM, et al. J Am Soc Nephrol 2017;28:632–44.
- Himmelfarb J, et al. Nat Rev Nephrol 2020;16(10):573-585.