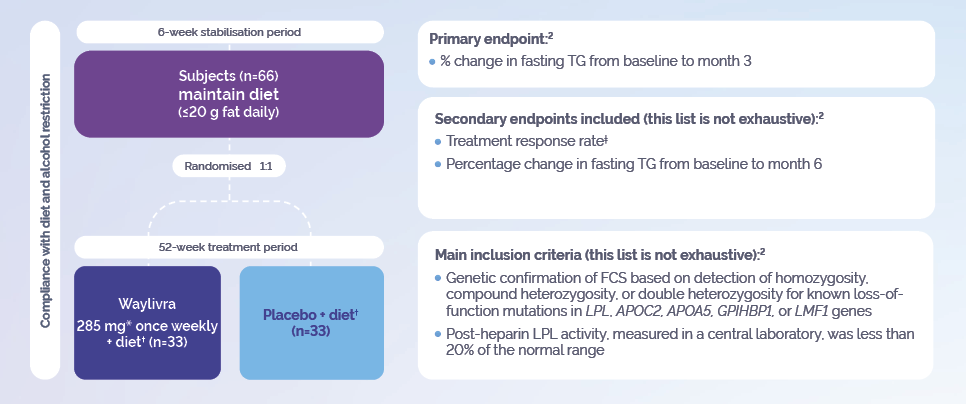
* The recommended starting dose is 285 mg in 1.5 ml injected subcutaneously once weekly for 3 months. Following 3 months, dose frequency should be reduced to 285 mg every 2 weeks.
† <20 grams of fat per day.
‡ Response was defined as a fasting plasma triglycercide level of less than 750 mg per deciliter at 3 months.
Adapted from Witzum JL et al. N Engl J Med 2019;381(6):531–42.
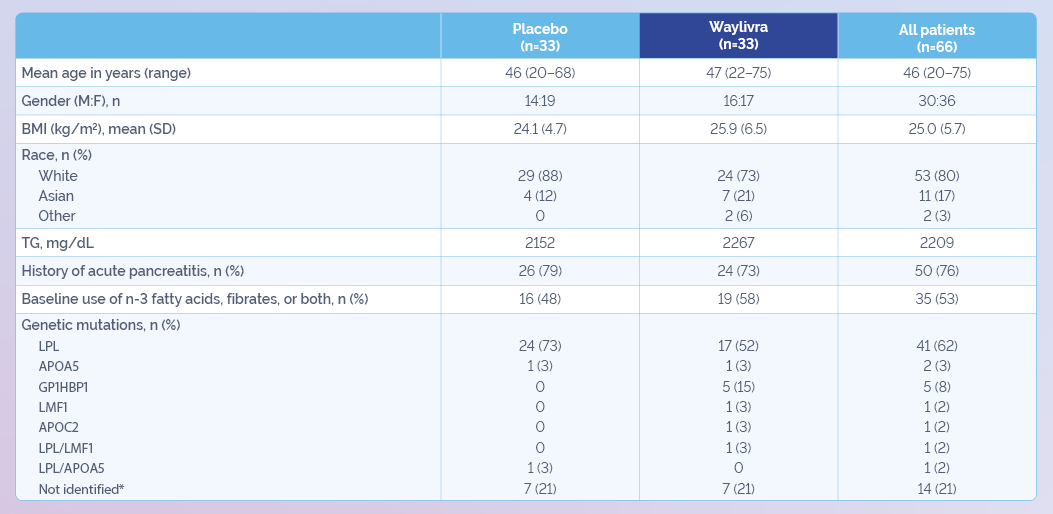
* Among 14 patients with unidentified genetic mutations, 6 had mutations in LPL or accessory proteins not annotated or predicted to be inactivating; 5 had no known relevant mutations. Genetic testing was not performed in three patients because of lack of consent – they were enrolled based on other criteria.
APOA5: apolipoprotein A5, APOC2: apolipoprotein C2, BMI: body mass index,FCS: familial chylomicronemia syndrome, GPIHBP1: glycosylphosphatidylinositol anchored high density lipoprotein binding protein 1, LMF1: lipase maturation factor 1, LPL: lipoprotein lipase, n: number of patients, SD: standard deviation, TG: triglycerides
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Swedish Orphan Biovitrum Ltd at [email protected] or Telephone +44 (0) 800 111 4754