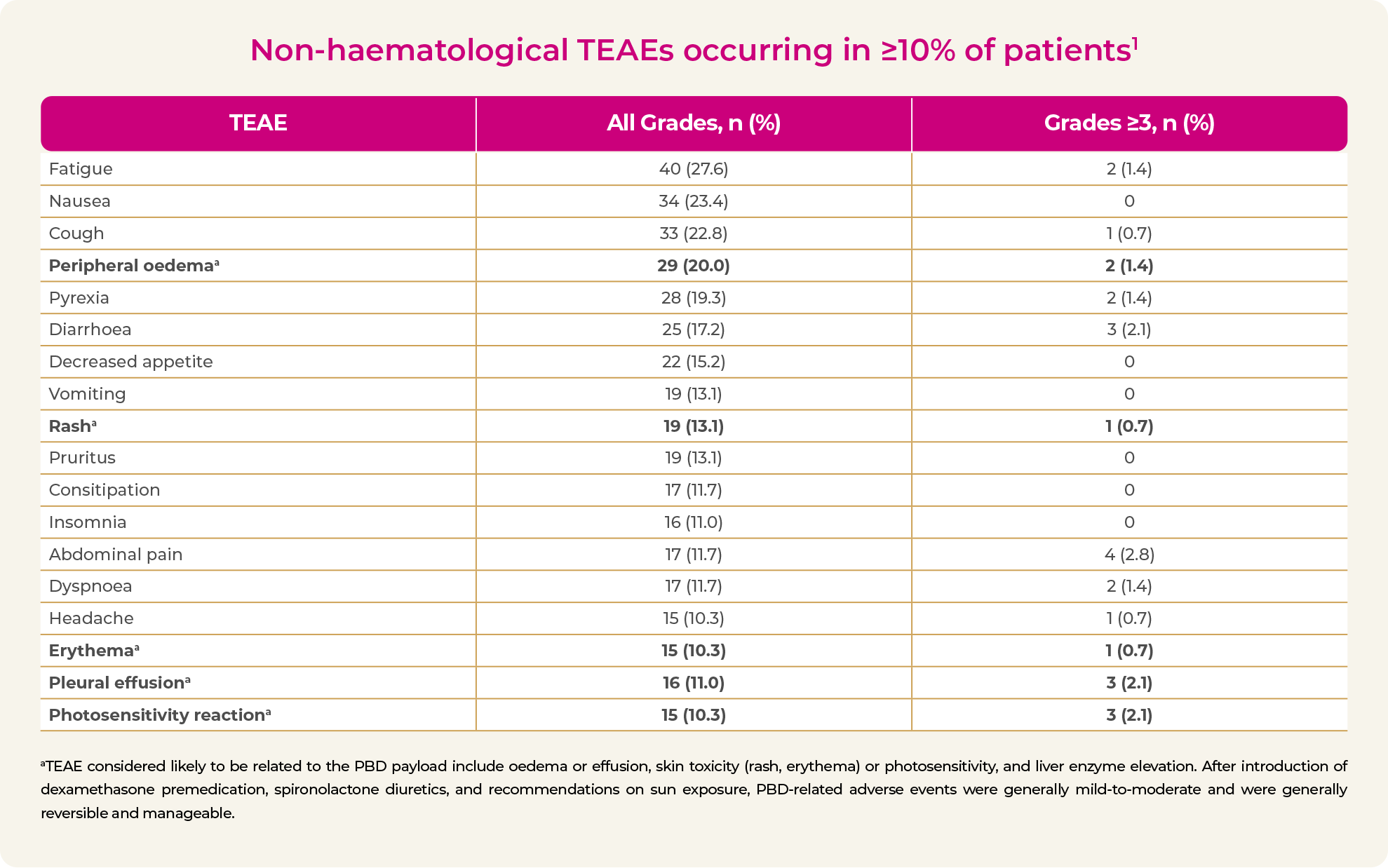
- Photosensitivity and cutaneous reactions have been reported with ZYNLONTA2,3
- Grade 3 reactions occurred in a total of 3.7% of patients in LOTIS-1 and LOTIS-2
- No Grade 4 or 5 events3
These adverse events can be minimised with actions such as patient education and using pre-treatment medication2
When prescribing ZYNLONTA, please ensure that you share the Patient Card (risk minimisation material) with your patients
Patient Card can be accessed on the ZYNLONTA EMC website or requested from your local Sobi representative

- Dexamethasone premedication
Advise patients to:
- Minimise or avoid exposure to natural and artificial sunlight, including through glass or car windows
- Protect skin with clothing and sunscreen products
- Use sunblock - even in cloudy or overcast conditions
- Always carry their Patient Alert Card for phototoxicity with them
- Report any skin reactions, such as: sensitivity to sunlight (including sunburn-like reactions); itchy rash; blistering; dark patches; irritation, swelling, pain, and/or skin damage at the injection site

- Monitor patients for new or worsening cutaneous reactions, including photosensitivity reactions
- Withhold ZYNLONTA for Grade ≥3 reactions until toxicity has resolved to Grade ≤1
- Oral/topical corticosteroids and anti-pruritic therapy was used in LOTIS-1 and LOTIS-2 to treat cutaneous reactions
- Consider dermatologic consultation if necessary
▼This medicine is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Swedish Orphan Biovitrum Ltd by email at [email protected] or by calling +44 (0) 800 111 4754.