Kineret is widely considered to be a well-tolerated treatment. The safety profile of Kineret has been well established in several placebo-controlled, randomised trials, as well as clinical use following its first marketing authorisation in 2002.1
The most common and consistently reported treatment-related adverse reaction associated with Kineret is injection site reactions (ISRs). The reactions are usually mild to moderate and take the form of redness, bruising, inflammation, pain, or discomfort at the injection site. ISRs typically appear within 2 weeks of starting Kineret and disappear within 4-6 weeks. ISRs are unlikely to occur if they have not appeared in the first month of treatment. These were characterised by 1 or more of the following: erythema, ecchymosis, inflammation, and pain.1,2
Very common adverse reactions include:1
- Redness, swelling, bruising, or itching at the injection site
Symptoms are generally mild to moderate and more common at the start of treatment.
- Headaches
- Increased total blood cholesterol levels.
Common adverse reactions include:1
- Neutropenia (low white blood cell count)
Determined after a blood test. This might increase the risk of getting an infection.
- Serious infections
Such as pneumonia (a chest infection) or infections of the skin.
- Thrombocytopenia (low level of blood platelets)
Uncommon adverse reactions include:1
- Elevated levels of liver enzymes
Determined after a blood test.
- Allergic reactions
Including anaphylactic reactions, angioedema, urticaria, rash, and pruritus.
Adverse reactions not known:1
- Injection site amyloid deposits
- Drug reaction with eosinophilia and systemic symtoms (DRESS)
During post-marketing use, drug reaction with eosinophilia and systemic symptoms (DRESS) has rarely been reported in patients treated with Kineret, predominantly in paediatric patients with Still's disease (systemic juvenile idiopathic arthritis (SJIA)).
Please refer to the SmPC section 4.8 for a full list of possible adverse reactions.
The short elimination half-life (median 5.7 hours) for Kineret allows for prompt dose adjustments or discontinuation if required.1,2
How to manage ISRs
The most common adverse reaction associated with Kineret is injection site reactions, mostly reported as mild to moderate. ISRs typically appear within two weeks of starting treatment and disappear within 4-6 weeks.1
The reactions are usually mild to moderate and take the form of redness, bruising, inflammation, pain, or discomfort in the area where the needle pricks the skin. Skin reactions are unlikely to occur if they have not appeared in the first month of treatment.1
For a more comfortable injection, the syringe can be taken out of the refrigerator 30 minutes before the injection to allow it to warm to room temperature. The pre-filled syringe can also be held gently in one hand, warming it for a few minutes. Kineret must not be warmed in any other way (for example, in a microwave or in hot water).1
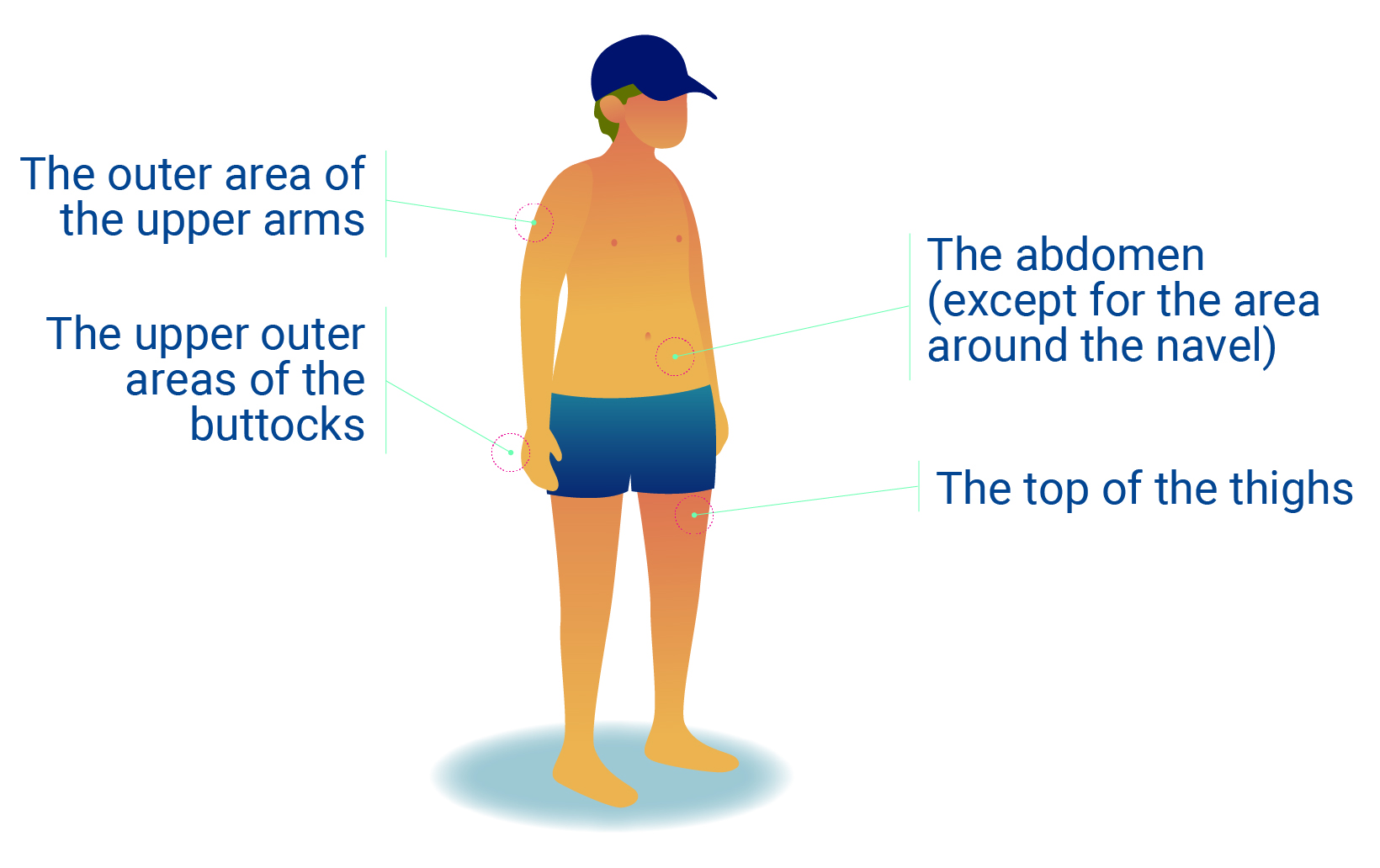
It is important to regularly change the place where the injection is administered so it doesn’t become sore in one area.4
Other suggestions for alleviating the signs and symptoms of ISRs include:
- Rotating injection sites, leaving at least an inch between injection sites.
- Cooling the injection site with an ice pack before and after injecting.1
Still’s Disease
The safety profile of Kineret in Still’s disease is consistent to that in patients with Cryopyrin-Associated Periodic Syndromes (CAPS) or Rheumatoid Arthritis (RA). The short half-life (median 5.7 hours) of Kineret makes it well suited for treatment of Still's disease if withdrawal of treatment is needed.1,2
Cryopyrin-Associated Periodic Syndromes (CAPS)
Kineret safety has been established in long-term daily-use, in patients with CAPS.5
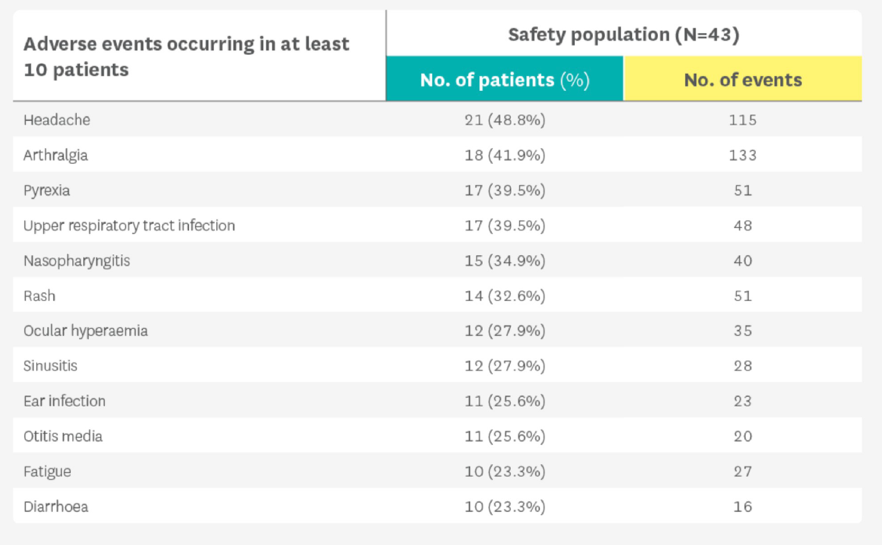
Adapted from Kullenberg t, et al. Rheumatology (Oxford). 2016;55(8):1499-1506.5
Safety data were taken from a published prospective cohort study of 43 patients treated with Kineret for up to five years between 2003 and 2010.5
Familial Mediterranean Fever (FMF)
The safety profile of Kineret in FMF is consistent to that in patients with Cryopyrin-Associated Periodic Syndromes (CAPS), Rheumatoid Arthritis (RA), or Still’s disease. The short half-life (median 5.7 hours) of Kineret may be an advantage allowing opportunity for more frequent dose tailoring and rapid withdrawal if necessary.1,2
Safety monitoring
Sobi is committed to monitoring the possible risks for adverse events or safety events with Kineret treatment. The risks are collected and assessed in a Risk Management Plan (RMP) which also provides information on how to minimise the risks.
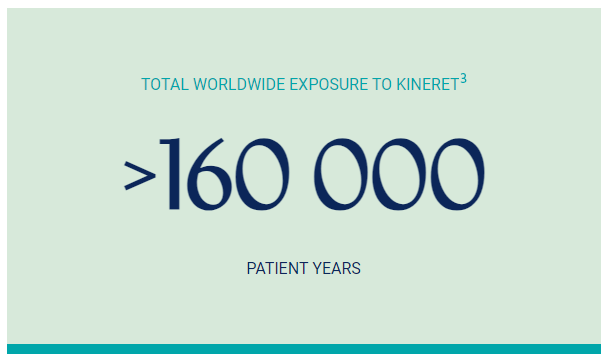
The identified safety risks with Kineret, as outlined in the RMP, comprise ISRs, immunogenicity, serious infections, neutropenia, allergic conditions, and hepatic disorders. Potential risks include malignancies, macrophage activation syndrome, medication errors including reuse of syringes, and pulmonary events such as interstitial lung disease, pulmonary hypertension, and alveolar proteinosis.3
You are asked to report any adverse events in accordance with local regulations.
Risk Minimisation Measures in the form of educational tools and materials are available and healthcare professionals can contact Sobi at [email protected] for request.
Prescribing Kineret
Administration
Kineret is administered by subcutaneous injection with a pre-filled syringe.1
Dosing
Still’s Disease & FMF
- The recommended dose for patient with Still’s disease weighing 50 kg or more is 100 mg administered once daily.1
- Patients weighing less than 50 kg should be dosed by body weight with a starting dose of 1-2 mg/kg administered once daily.1
CAPS
- Starting dose:1
- The recommended dose in all CAPS subtypes is 1-2 mg/kg administered once daily by subcutaneous injection. The therapeutic response is primarily reflected by a reduction in clinical symptoms such as fever, rash, joint pain, and headache, but also in inflammatory serum markers (CRP/SAA levels), or occurrence of flares.
- Maintenance dose:1
- Mild CAPS (FCAS, mild MWS): Patients are usually well-controlled by maintaining the recommended starting dose (1-2 mg/kg once daily).
- Severe CAPS (MWS and NOMID/CINCA): The usual maintenance dose is 3-4 mg/kg once daily, which can be adjusted to a maximum of 8mg/kg once daily. Dose increases may become necessary within 1 to 2 months based on therapeutic response.
Monitoring
Still’s Disease
Response to treatment should be evaluated after 1 month. In case of persistent systemic manifestations, dose may be adjusted in children, or continued treatment with Kineret should be reconsidered by the treating physician.1
CAPS
In addition to the evaluation of clinical symptoms and inflammatory markers in severe CAPS, assessments of inflammation of the CNS, including the inner ear (MRI or CT, lumbar puncture, and audiology) and eyes (ophthalmological assessments) are recommended after an initial 3 months of treatment, and thereafter every 6 months, until effective treatment doses have been identified. When patients are clinical well-controlled, CNS and ophthalmological monitoring may be conducted yearly.1
Injection
- Alternating the injection site is recommended to avoid discomfort at the site of injection.1
CAPS, Cryopyrin-Associated Periodic Syndromes; CINCA, Chronic Infantile Neurological Cutaneous and Articular syndrome; CNS, central nervous system; CRP, C-reactive protein; CT, computerised tomography; FCAS, Familial Cold Autoinflammatory Syndrome; FMF, Familial Mediterranean fever; IL, interleukin; ISR, injection site reaction; MRI, magnetic resonance imaging; MWS, Muckle-Wells Syndrome; NOMID, Neonatal-Onset Multisystem Inflammatory Disease; RA, rheumatoid arthritis; RMP, Risk Management Plan; SAA, serum amyloid A protein; SmPC, Summary of Product Characteristics.
References
- Kineret Summary of Product Characteristics. https://www.medicines.org.uk/emc/product/559/smpc (Accessed December 2025).
- EMA. Kineret: EPAR - CHMP extension of indication variation assessment report. Available from: https://www.ema.europa.eu/en/documents/variation-report/kineret-h-c-363-ii-0056-epar-assessment-report-variation_en.pdf (Accessed December 2025).
- EMA. Kineret: EPAR - Risk-management plan. Available from: https://www.ema.europa.eu/en/documents/rmp-summary/kineret-epar-risk-management-plan_en.pdf (Accessed December 2025).
- Kineret Patient Information Leaflet. Available at: https://www.medicines.org.uk/emc/files/pil.559.pdf (Accessed December 2025).
- Kullenberg T, et al. Rheumatology. 2016;55(8):1499–1506.
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard or search MHRA Yellow Card in the Google Play or Apple App Store (for United Kingdom) and www.hpra.ie (for Republic of Ireland). Adverse events should also be reported to Swedish Orphan Biovitrum Ltd at [email protected] or Telephone +44 (0) 800 111 4754



