FMF
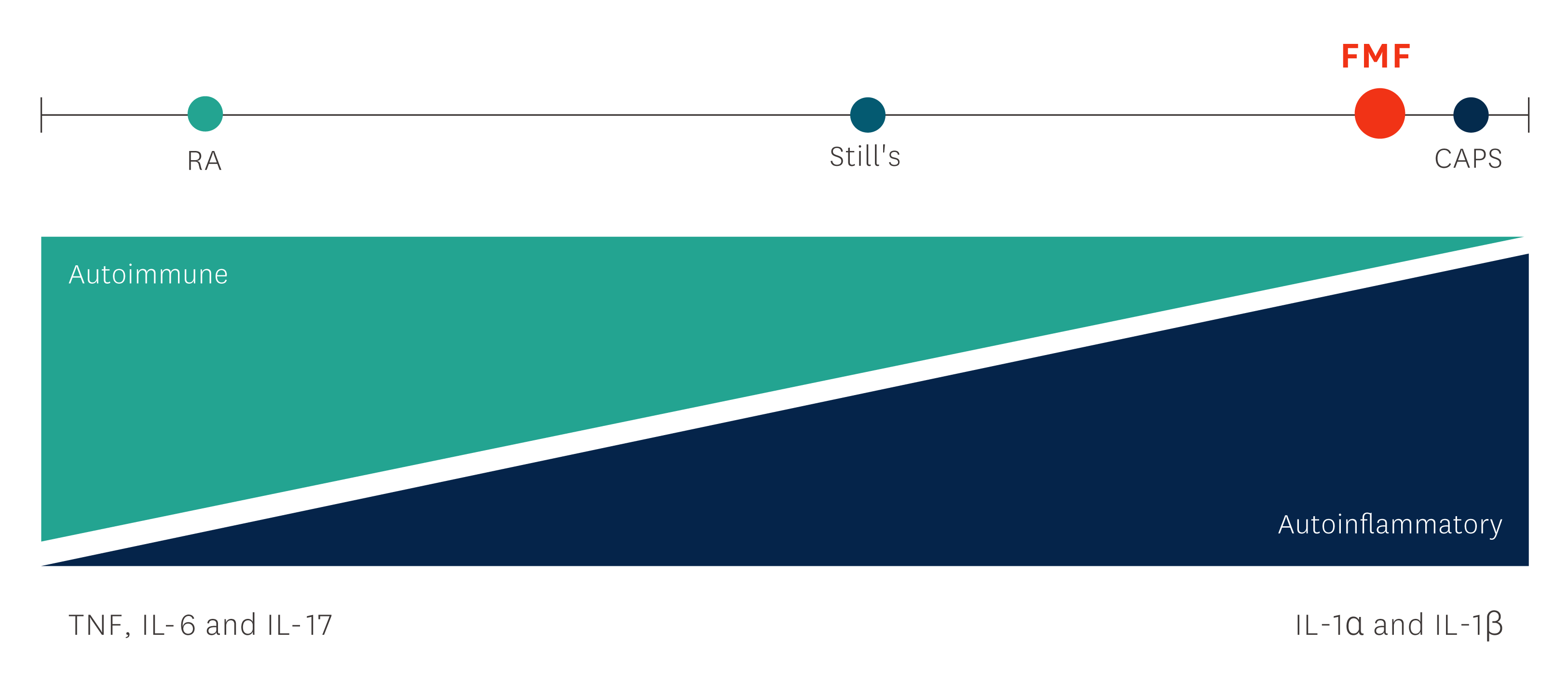
Familial Mediterranean fever (FMF) is the most common inherited monogenic autoinflammatory disease and one of the periodic fever syndromes, which mainly affects ethnic groups from the eastern Mediterranean area.1
The following sections include further background information on this condition, introduce the proven efficacy and safety of Kineret in patients with FMF, and provide appropriate guidance on dosing and administration of Kineret.
Disease background
Familial Mediterranean Fever (FMF) is the most common inherited monogenic autoinflammatory disease and affects mainly ethnic groups from the eastern Mediterranean area. FMF is one of the periodic fever syndromes.1
The disease is characterised by self-limiting periodic episodes of fever, joint pain and serositis along with elevated acute phase markers such as ESR, SAA and CRP.1, 2
FMF is caused by genetic mutations in specific loci which result in increased production of IL-1β and thereby increased inflammatory activity. Patients with untreated FMF are at risk of developing amyloidosis with renal insufficiency.1- 3

Onset: <20 years of age in 90% of patients. Mean age of onset: 3 to 9 years; onset during the first year of life is not as common as in other autoinflammatory diseases4
Efficacy
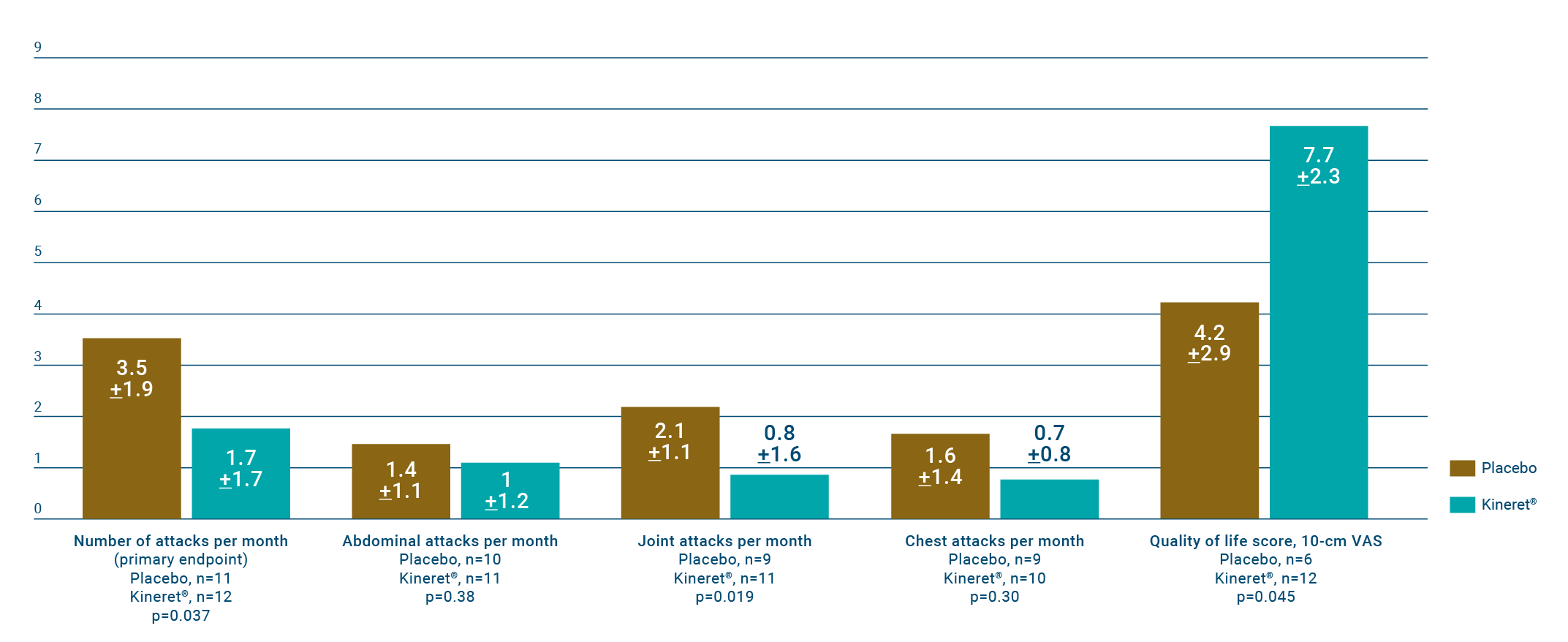
Treatment with Kineret reduced the number of attacks per month and significantly improved patients' quality of life scores.6
A single-centre, randomised, placebo-controlled study evaluated the efficacy and safety of Kineret in the treatment of colchicine-resistant FMF over a period of 4 months.6
Patients
25 patients with colchicine-resistant FMF were enrolled, of whom 12 were randomised to receive Kineret and 13 to receive placebo.
Primary endpoint
To compare the difference between the Kineret and placebo groups in the total number of attacks over the study period, adjusted to participation time (number of attacks, per patient per month), as well as the difference between the Kineret and placebo groups in the number of patients with a mean of <1 FMF attack per month.
Key definitions of response characterisation
Improvement according to the modified FMF50 score in each group was determined based on the presence of ≥ 3 of the 4 following items: ≥ 50% decrease in the frequency of all attacks; ≥50% decrease in the frequency of attacks in the joints; ≥50% decrease in the levels of CRP or SAA levels (or normalisation of these markers, defined as ≤ 5 mg/L for CRP and ≤10 mg/L for SAA); ≥50% increase in QoL values. FMF attacks were considered if they met the following criteria: fever of ≥38°C lasting 6 hours to 7 days and accompanied by painful manifestations in either the abdomen, the chest, the joints, or the skin.
Patient baseline characteristics⁶
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
† Tachycardia, paroxysmal atrial fibrillation, transient ischemic attack. ‡ Status post bacterial endocarditis and aortic regurgitation, episodic vertigo, panic attacks.
The mean number of attacks per patient/month was 1.7 ± 1.7 in those receiving Kineret compared to 3.5 ± 1.9 in the placebo group (n=25, P = 0.037).
Six patients in the Kineret group had < 1 attack/month, compared to none in the placebo group (P = 0.005).
The safety outcomes were similar between the two patient groups, including the number and rate of AEs, associations of AEs with specific organ systems, and associations of AEs with the study drug and severity. No SAEs were recorded, and none of the AEs were graded as severe.
AEs, adverse events; CAPS, Cryopyrin-Associated Periodic Syndromes; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; FMF, familial Mediterranean fever; FMF50, familial Mediterranean fever 50 score; GI, gastrointestinal; IL, interleukin; QoL, quality of life; RA, rheumatoid arthritis; SAA, serum amyloid A; SAE, serious adverse event; TNF, tumour necrosis factor; VAS, visual analogue score.
References
- Özen S, et al. Front Immunol . 2017;8:253.
- Padeh S, et al. Familial Mediterranean fever. In: Hashkes PJ, et al. (eds). Textbook of Autoinflammation. Springer; 2019. p293–314.
- Ben-Chetrit E, Touitou I. Arthritis Rheum . 2009;61(10):1447–53.
- Ozdogan H, Ugurlu S. Presse Med . 2019;48:e61–e76.
- McGonagle D, McDermott MF. Plos Med. 2006;3(8):e297.
- Ben-Zvi I, et al. Rheumatol . 2017;69(4):854–62.
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard or search MHRA Yellow Card in the Google Play or Apple App Store (for United Kingdom) and www.hpra.ie (for Republic of Ireland). Adverse events should also be reported to Swedish Orphan Biovitrum Ltd at [email protected] or Telephone +44 (0) 800 111 4754