Dosing Information
Waylivra is the only medicine licensed* and recommended by NICE for the treatment of FCS.1,2
The recommended starting dose is 285 mg in 1.5 ml injected subcutaneously once weekly for 3 months.1
Following 3 months, dosing frequency should be adjusted based on your patients’ response to treatment.1
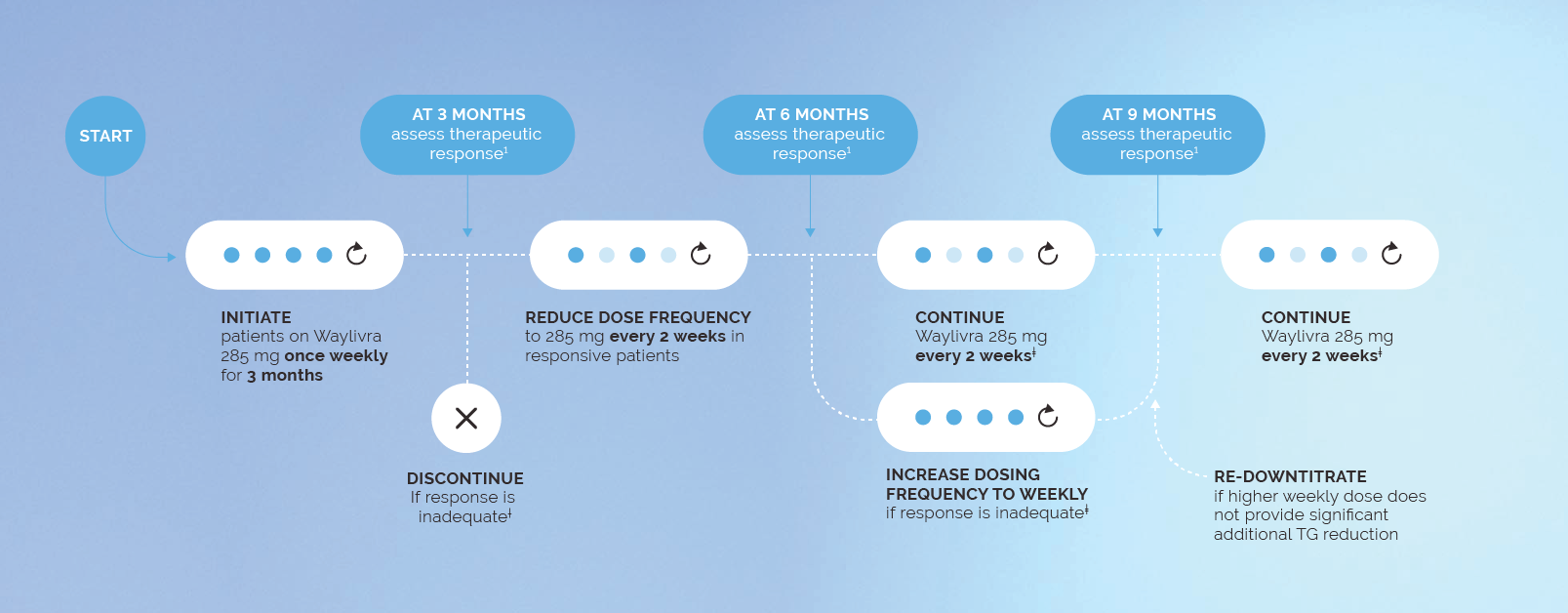
Treatment should be initiated by and remain under the supervision of a physician experienced in the treatment of patients with FCS.1 Prior to initiating Waylivra, the platelet count should be measured,§ and secondary causes of hypertriglyceridemia (e.g. uncontrolled diabetes, hypothyroidism) should be excluded or appropriately addressed.1 For effective TG control, patients should also maintain the FCS-specific low-fat diet alongside Waylivra.1
Please consult the SmPC for full dosing information.
* Waylivra is licensed under a conditional marketing authorisation.
† Inadequate response defined as a reduction in serum TG <25% or failure to achieve serum TG <22.6 mmol/L after 3 months on Waylivra 285 mg weekly.1
‡ Provided platelet counts are normal.
§ If the platelet count is below 140 x 109/L, another measurement should be taken approximately a week later to reassess. If platelet count remains below 140 x 109/L upon a second measurement, Waylivra should not be initiated (see section 4.3 of the SmPC).1
FCS: familial chylomicronemia syndrome, NICE: National Institute for Health and Care Excellence, SmPC: Summary of Product Characteristics; TG: triglycerides
References
- Waylivra Summary of Product Characteristics.
- ©NICE 2020 Volanesorsen for treating familial chylomicronaemia syndrome. Available from www.nice.org.uk/guidance/hst13. All rights reserved. Subject to notice of rights. (Accessed March 2025).
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Swedish Orphan Biovitrum Ltd at [email protected] or Telephone +44 (0) 800 111 4754



