Risk Management Materials
Educational Risk Management Materials to help reduce the risk associated with using this medicine, including a patient and carer guide and a risk management healthcare professional guide, are available as part of the regulatory requirement of Waylivra at: https://www.medicines.org.uk/emc/product/10636/rmms/.
Thrombocytopenia
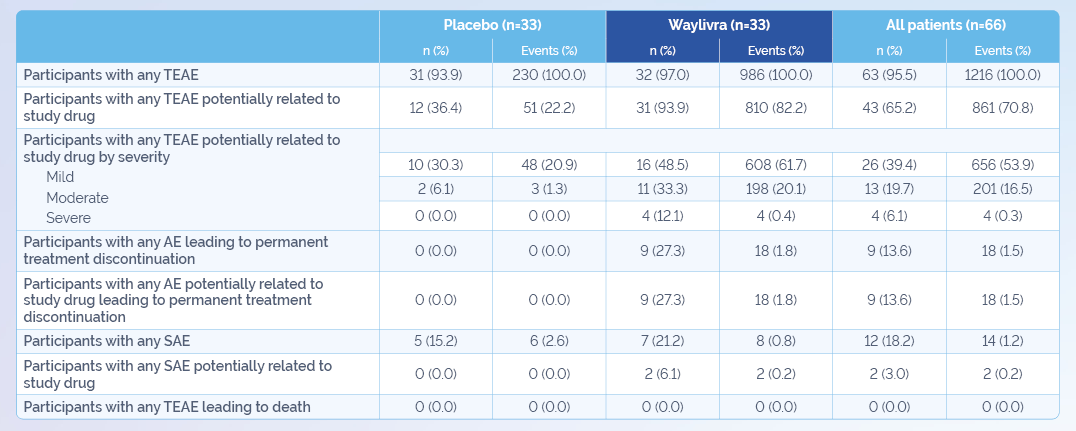
Waylivra is associated with reductions in platelet count.1 Patients should be monitored for thrombocytopenia during treatment.1
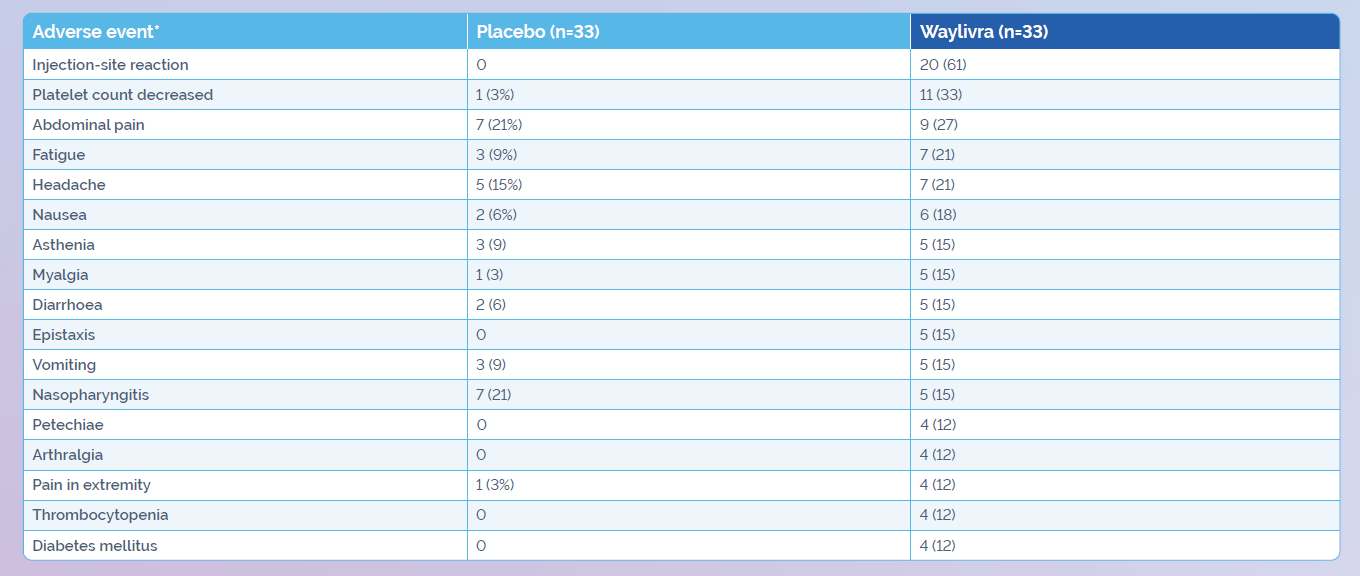
Adverse events reported during the APPROACH study:1
- Platelet counts below 140 x 109/L and 100 x 109/L were observed in 76% and 47% of Waylivra-treated patients, respectively
- Decreased platelet count was reported as an adverse event in 11 (33%) of Waylivra-treated patients
versus 1 (3%) of placebo-treated patients
- Thrombocytopenia was reported in 4 (12%) of Waylivra-treated patients vs none in the placebo group
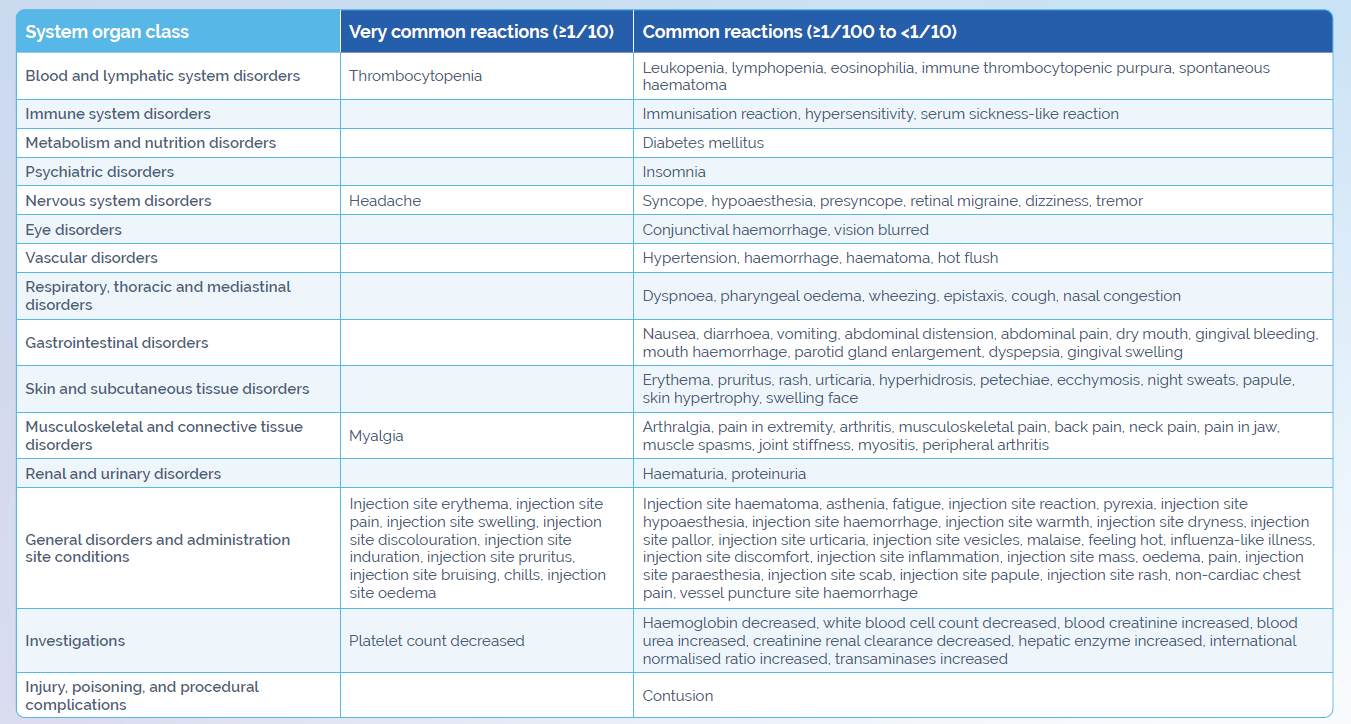
Always consult the SmPC for further information on Waylivra’s safety profile
Adapted from Waylivra SmPC.
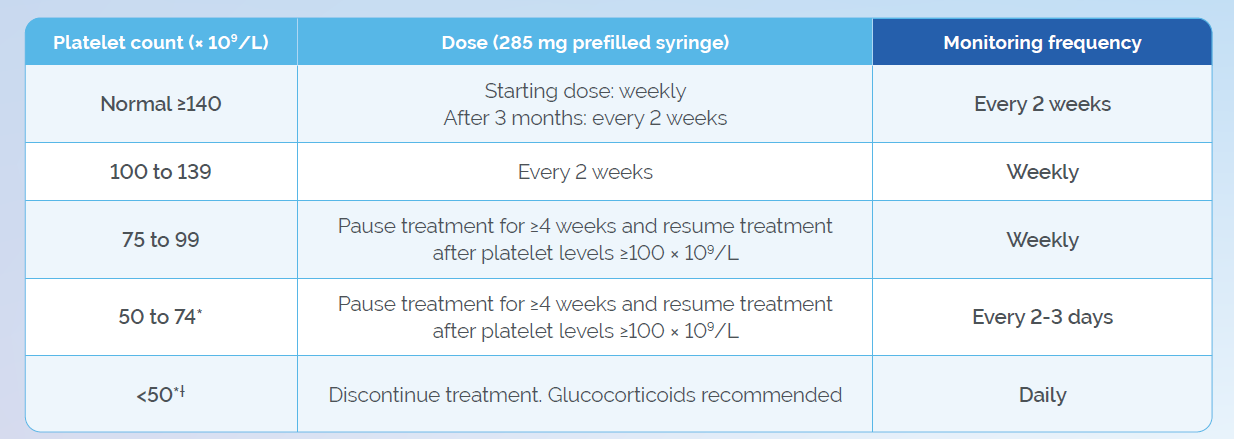
* Discontinuation of antiplatelet medicinal products/NSAIDs/anticoagulants should be considered for platelet levels <75 × 109/L. Treatment with these medicinal products must be discontinued at platelet levels <50 × 109/L.1
† Consultation of a haematologist is needed to reconsider the benefit/risk for possible further treatment with Waylivra in a patient who has discontinued due to severe thrombocyotpenia (<50 x 109/L).1
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Swedish Orphan Biovitrum Ltd at [email protected] or Telephone +44 (0) 800 111 4754