Cryopyrin-Associated Periodic Syndromes (CAPS)
CAPS are a spectrum of autoinflammatory disorders related to defects in the protein cryopyrin (also called NLRP3) and include familial cold autoinflammatory syndrome (FCAS), Muckle-Wells syndrome (MWS) and neonatal onset multisystem inflammatory disease (NOMID)/chronic inflammatory neurological cutaneous articular syndrome (CINCA).1
Kineret is indicated for the treatment of CAPS, including:2
- Neonatal-Onset Multisystem Inflammatory Disease (NOMID) / Chronic Infantile Neurological, Cutaneous, Articular Syndrome (CINCA)
- Muckle-Wells Syndrome (MWS)
- Familial Cold Autoinflammatory Syndrome (FCAS)
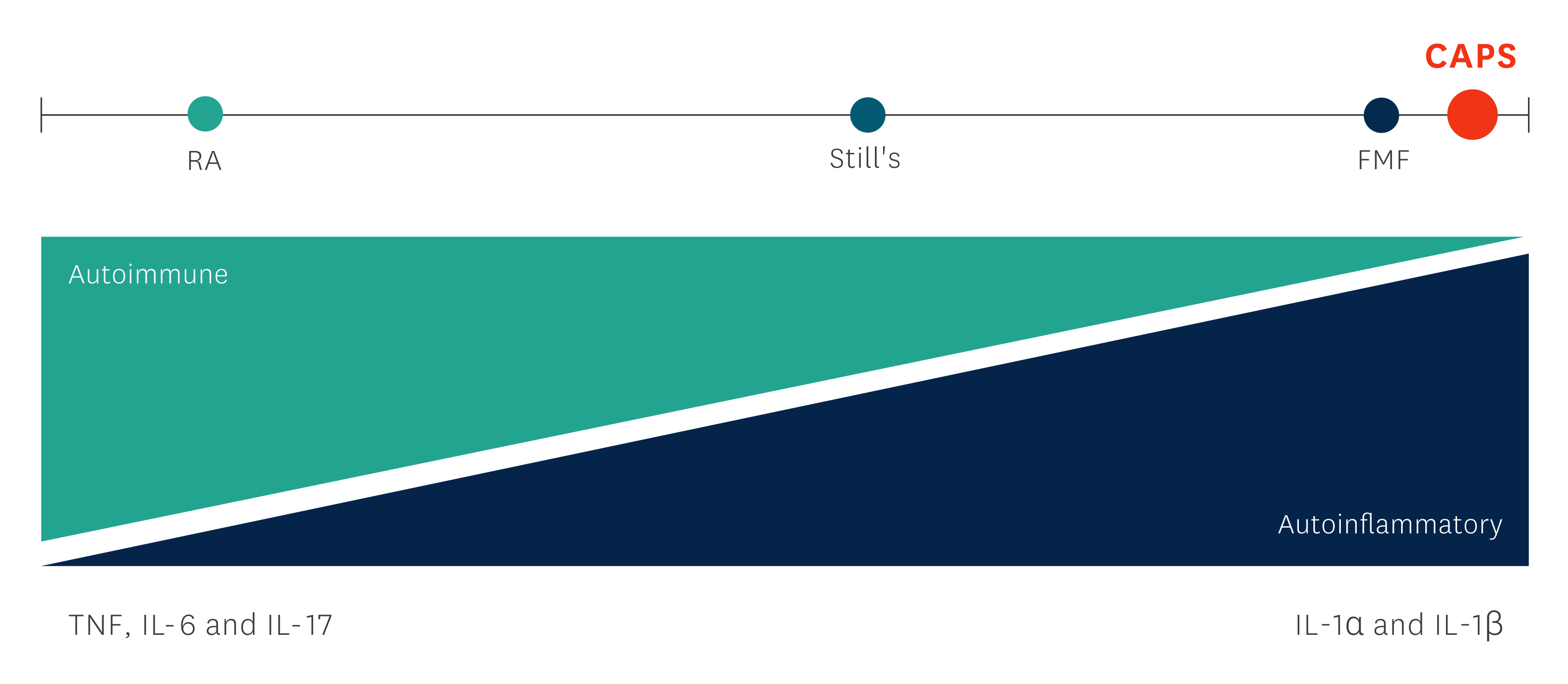
Diagram adapted from: Figure 1, McGonagle D, McDermott MF. PLos Med. 2006;3(8):e297.3
The following sections include further background information on this condition, introduce the proven efficacy and safety of Kineret in patients with CAPS, and provide appropriate guidance on dosing and administration of Kineret.
Disease background
CAPS are lifelong, rare disorders that can manifest as serious health problems.1
Efficacy
Kineret provides rapid resolution of CAPS symptoms, with withdrawal uniformly resulting in relapse within days.5
One study looked at patients with active NOMID who were treated with Kineret, which was then withdrawn in some patients until a flare occurred. Due to the severity of the flares, the bioethics committee recommended discontinuation of the withdrawal phase.5
Patients
18 patients with active NOMID disease, despite treatment with NSAIDs and DMARDs or corticosteroids.
Primary endpoint
A change in the disease-specific daily diary score, and changes in the acute-phase reactants (SAA, CRP and the ESR) from baseline to three months and from three months until a flare up occurred.
Secondary endpoint
Childhood health assessment questionnaires, audiography, and vision evaluations were performed at baseline and at follow-up at one, three, and six months. All patients underwent a lumbar puncture at baseline and at three months.
Key definitions of response characterisation
Disease-specific diary scores were rated on a scale of 0 to 4 for increasing severity for each of the following symptoms: fever, rash, headache, joint pain and vomiting. Flare up defined as at least two of the following criteria: an increase in the rash score for four days, a temperature > 37°C on four or more occasions, vomiting or headache for three days, or a worsening of any neurosensory symptom.
Patient baseline characteristics
Patient groups were comparable at baseline except for the prevalence of hearing loss and brain MRI abnormalities.5
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
*Plus–minus values are means ±SD. Percentages may not total 100 because of rounding. †Race was self-reported by the patient. ‡Intracranial pressures were obtained for 14 patients at baseline. §Cerebrospinal-fluid cell counts were obtained for 15 patients at baseline. ¶Cognitive function was assessed with the use of the following age-appropriate standardized tests among 17 English-speaking patients: Wechsler Preschool and Primary Scale of Intelligence — Third Edition (4 patients), Wechsler Intelligence Scale for Children — Fourth Edition (8 patients), Wechsler Adult Intelligence Scale — Third Edition (3 patients), and the Vineland Adaptive Behaviour Scales — Interview Edition (2 patients). ||All patients had at least one abnormality on MRI. **Two additional patients had ventriculoperitoneal shunts. †† Fifteen patients had FIESTA sequences.
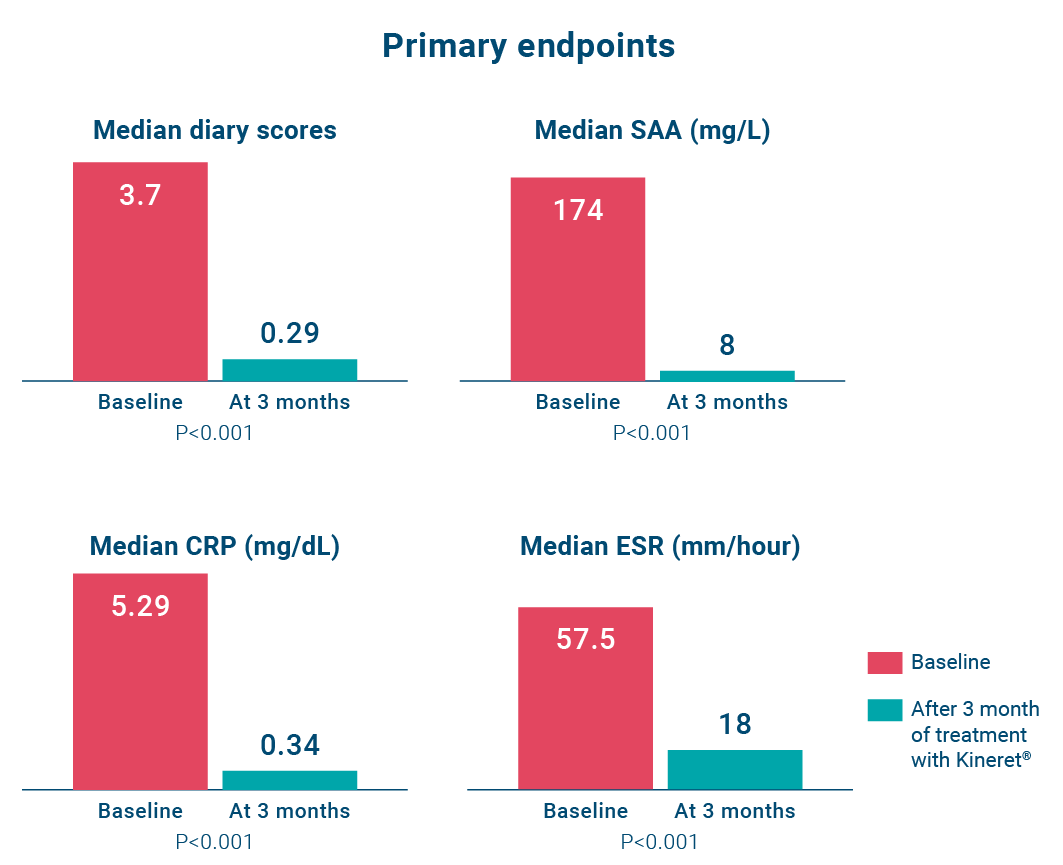
Kineret improved diary score and acute phase reactants (serum amyloid A, C-reactive protein, and the erythrocyte sedimentation rate) levels in patients with NOMID (n=18) compared to baseline over a 3-month treatment period.5
The diary scores significantly decreased at three months. Levels of serum amyloid A, C-reactive protein and the erythrocyte sedimentation rate all fell significantly with treatment in all patients.5
Diary scores improved (P<0.001) and serum amyloid A (from a median of 174 mg to 8 mg per liter), C-reactive protein (from a median of 5.29 mg to 0.34 mg per deciliter), and the erythrocyte sedimentation rate decreased at month 3 (all P<0.001), and remained low at month 6.5
Kineret significantly decreased major organ manifestations in patients with neonatal-onset multisystem inflammatory disease, by reducing the signs of conjunctivitis and cochlear enhancement.5
After three months of treatment, 11 patients underwent withdrawal of Kineret for a maximum of seven days. All but one patient fulfilled prespecified criteria for a flare of disease.
Patients had a response promptly after resuming Kineret, and improvements were sustained at the six-month follow-up evaluation.
In the study, none of the patients discontinued drug treatment. A localised, erythematous, and sometimes painful skin reaction at the injection site developed in eight patients (44%) and had disappeared in all patients at six weeks. Adverse events during treatment included upper respiratory infections (in 15 patients), urinary tract infections (in 2), and a hospital admission for dehydration from nonbacterial diarrhoea (in 1).
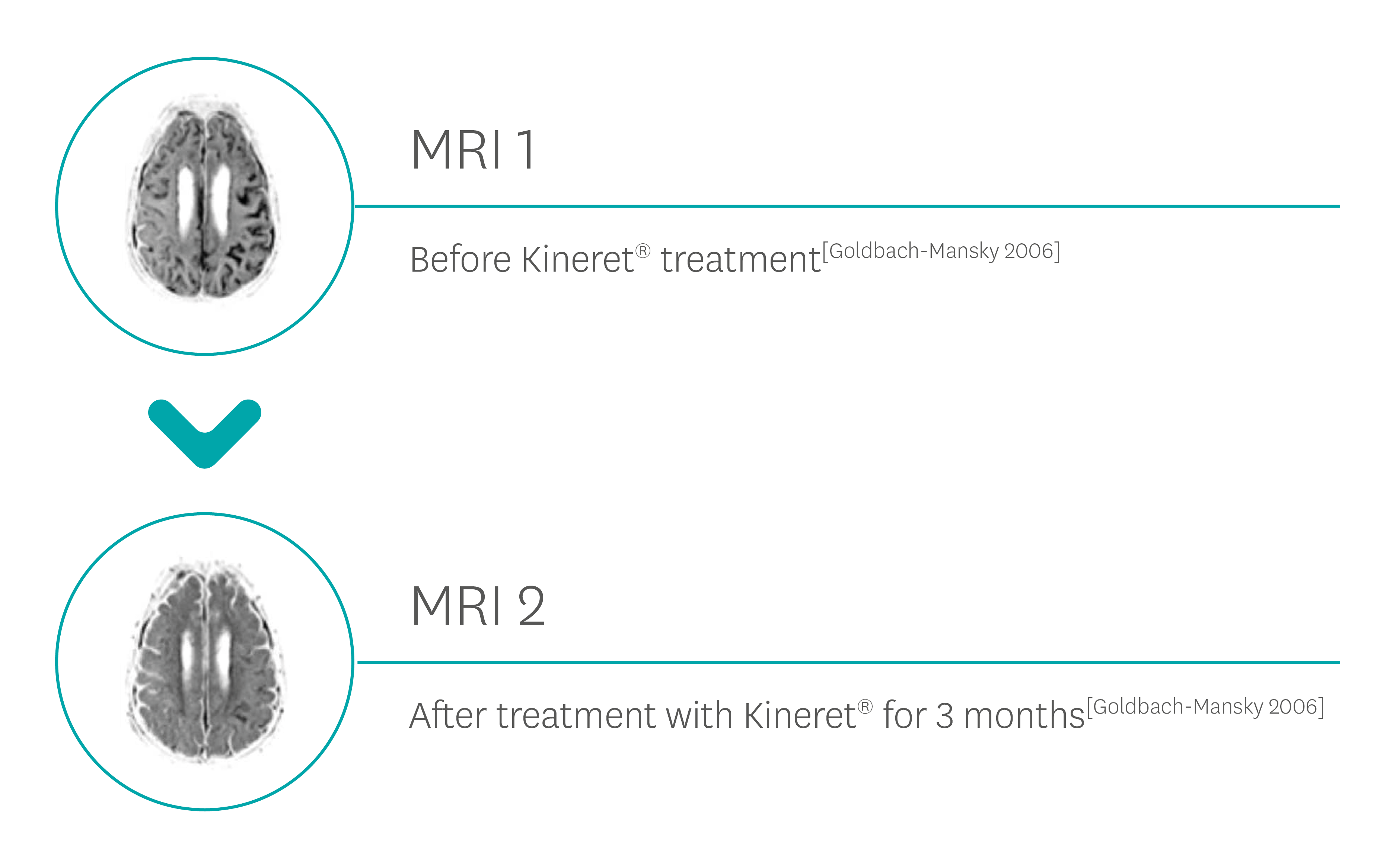
In the same study, rash and conjunctivitis resolved within three days in all patients, with 13/17 patients with cochlear enhancement at initial MRI showing either a reduction or complete resolution after 3 months of therapy.
CAPS, Cryopyrin-Associated Periodic Syndromes; CINCA, Chronic Infantile Neurological Cutaneous and Articular syndrome; CRP, C-reactive protein; DMARD, disease-modifying antirheumatic drug; ESR, erythrocyte sedimentation rate; FCAS, Familial Cold Autoinflammatory Syndrome; FIESTA, fast imaging employing steady-state acquisition; FMF, Familial Mediterranean fever; IL, interleukin, MRI, magnetic resonance imaging; MWS, Muckle-Wells Syndrome; NOMID, Neonatal-Onset Multisystem Inflammatory Disease; NSAID, nonsteroidal anti-inflammatory drug; RA, rheumatoid arthritis; SAA, serum amyloid A protein; TNF, tumour necrosis factor.
References:
- Kuemmerle-Deschner JB, et al. Ann Rheum Dis . 2017;76(6):942–7.
- Summary of Product Characteristics. https://www.medicines.org.uk/emc/product/559/smpc (Accessed May 2025).
- McGonagle D, McDermott MF. Plos Med. 2006;3(8):e297.
- Landmann EC, Walker UA. Expert Rev Clin Pharmacol . 2017;10(8):855–64.
- Goldbach-Mansky R, et al. N Engl J Med . 2006;355(6):581–92.
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard or search MHRA Yellow Card in the Google Play or Apple App Store (for United Kingdom) and www.hpra.ie (for Republic of Ireland). Adverse events should also be reported to Swedish Orphan Biovitrum Ltd at [email protected] or Telephone +44 (0) 800 111 4754