▼This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard (for United Kingdom) and www.hpra.ie (for Republic of Ireland). Adverse events should also be reported to Swedish Orphan Biovitrum Ltd at [email protected] or Telephone +44 (0) 800 111 4754.
Abbreviations:
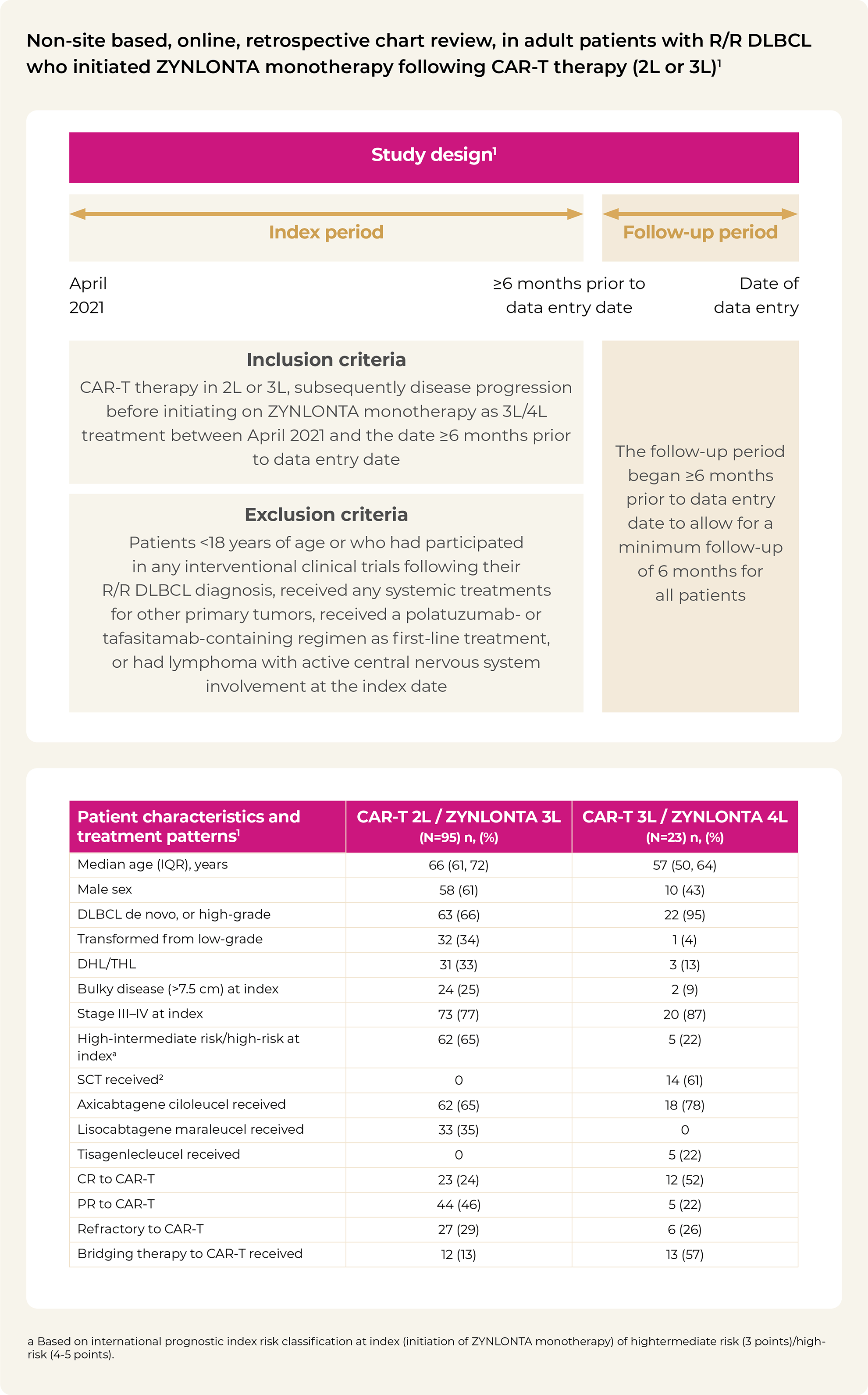
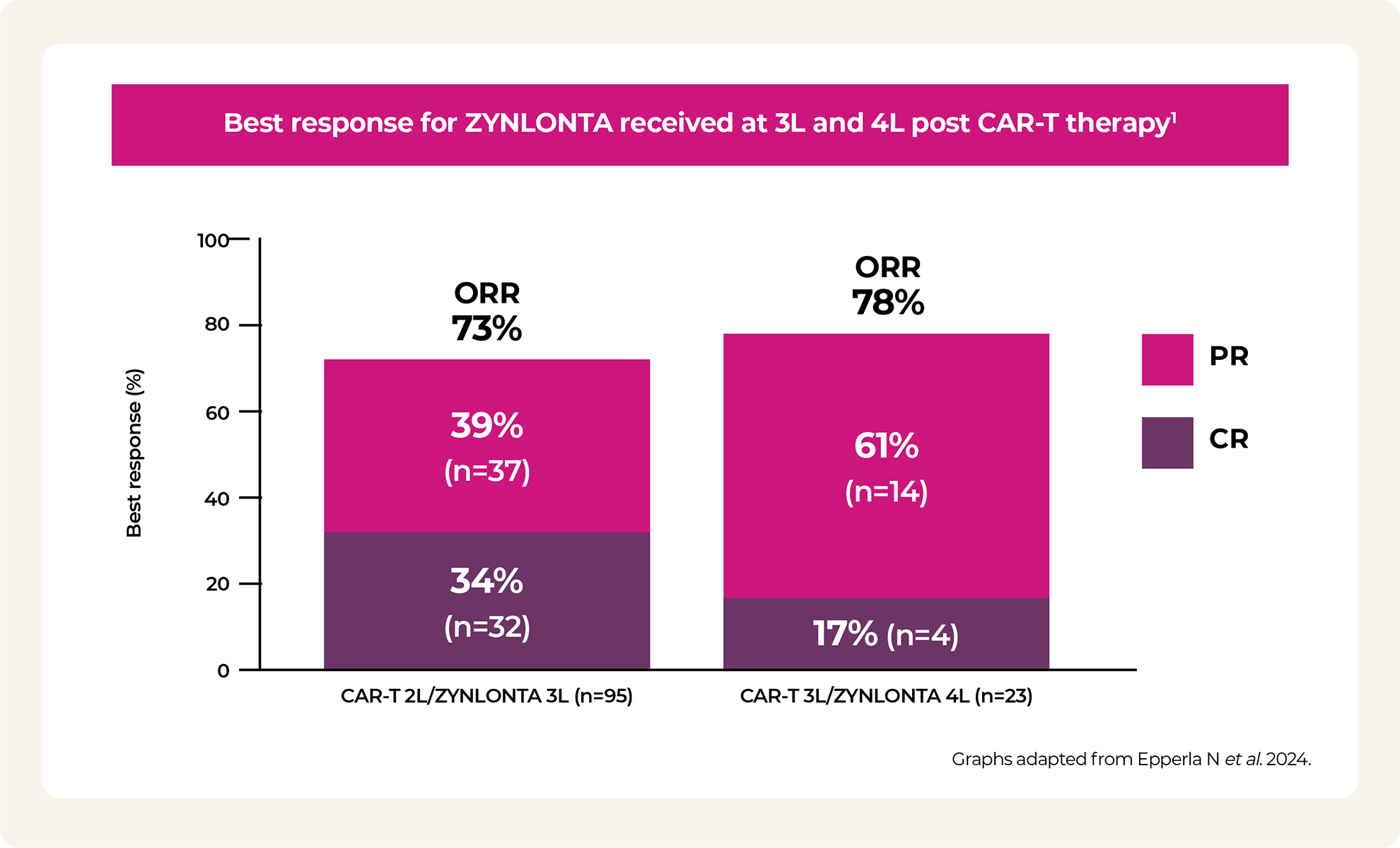
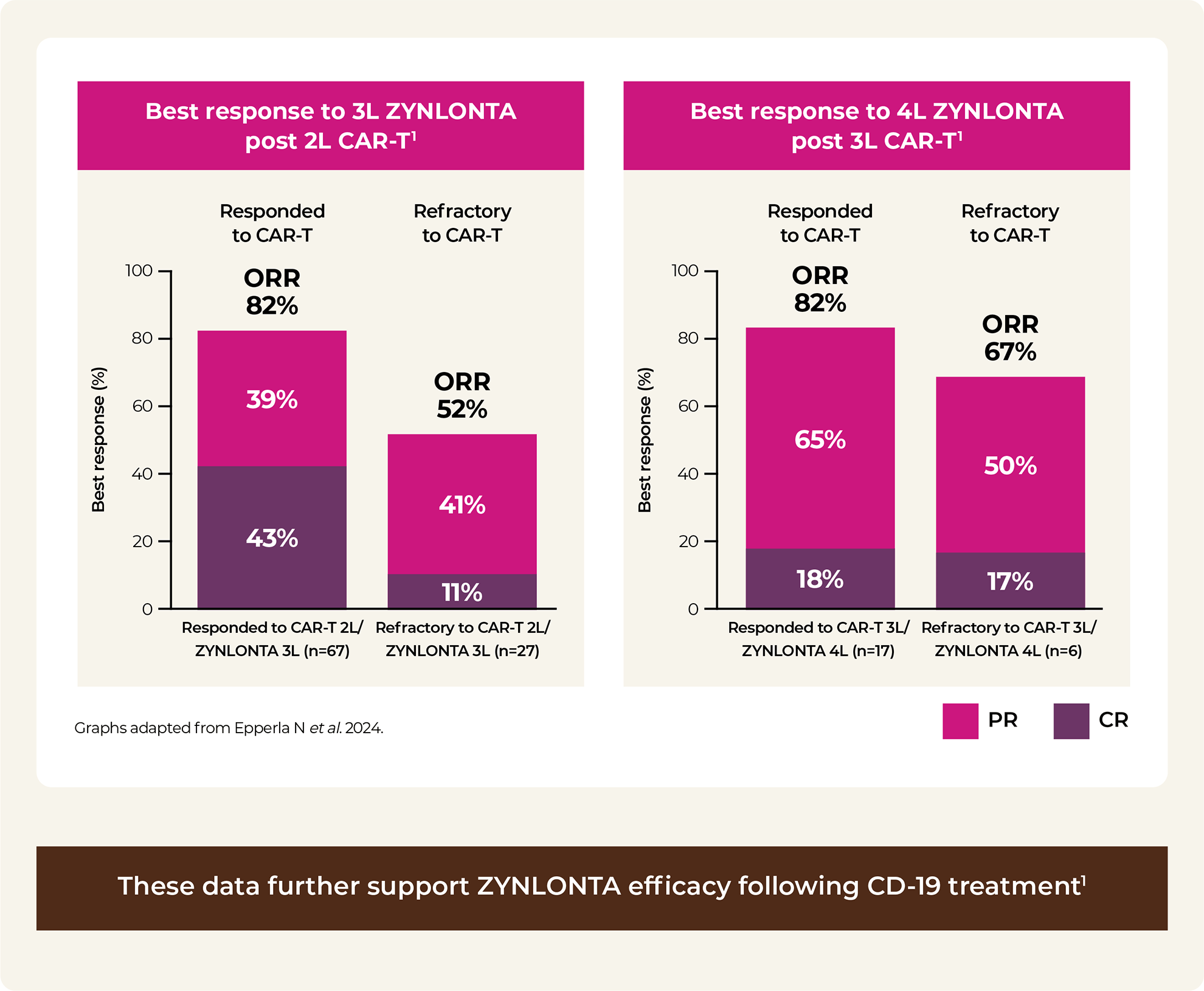
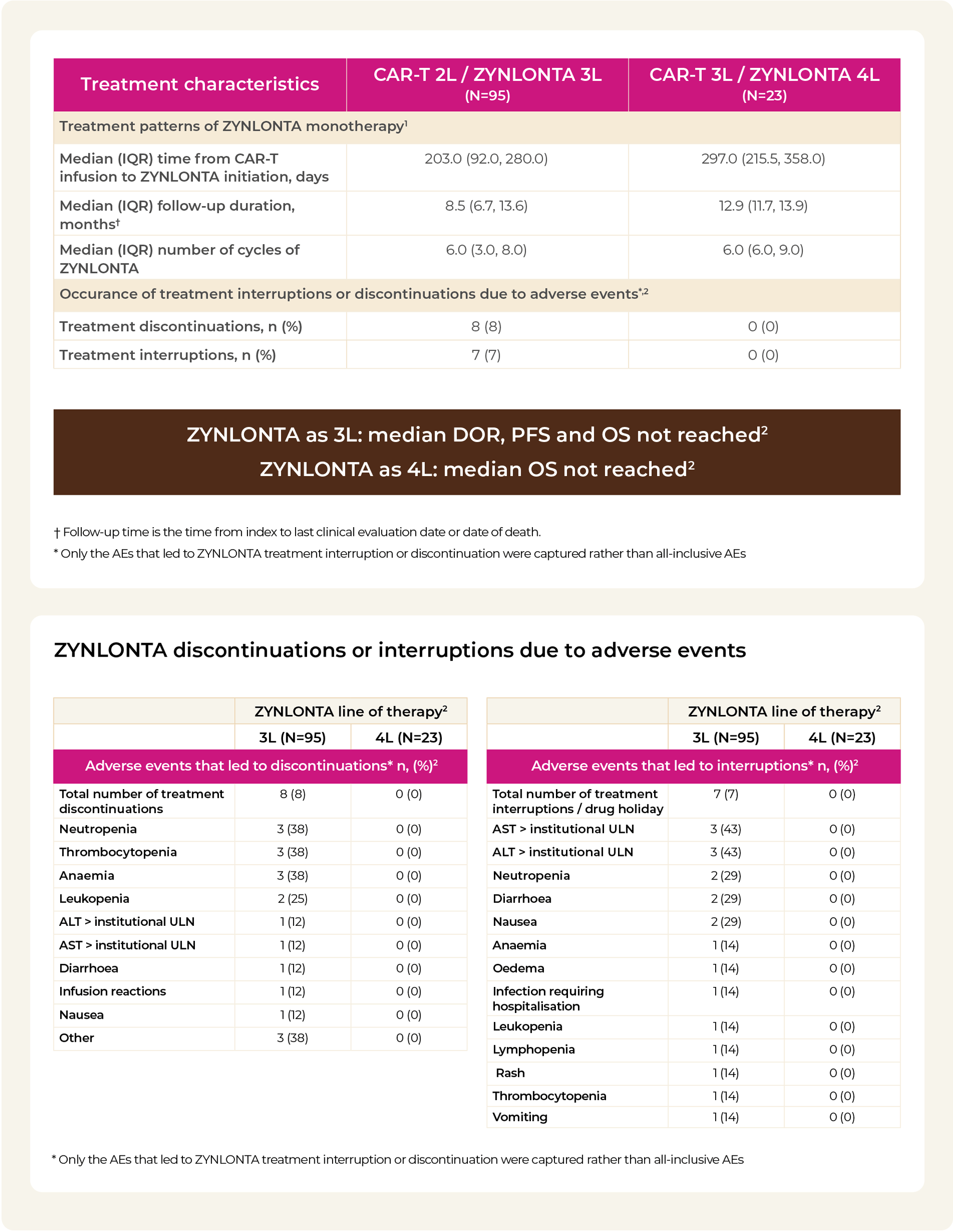
2L / 3L / 4L: second line, third line, fourth line; AEs: adverse events; ALT: alanine aminotransferase; AST: aspartate aminotransferase; CAR-T: chimeric antigen receptor T-cell; CR: complete response; DOR: duration of response; IQR: interquartile range; ORR: overall response rate; OS: overall survival; PR: partial response; PFS: progression-free survival; ULN: upper limit of normal