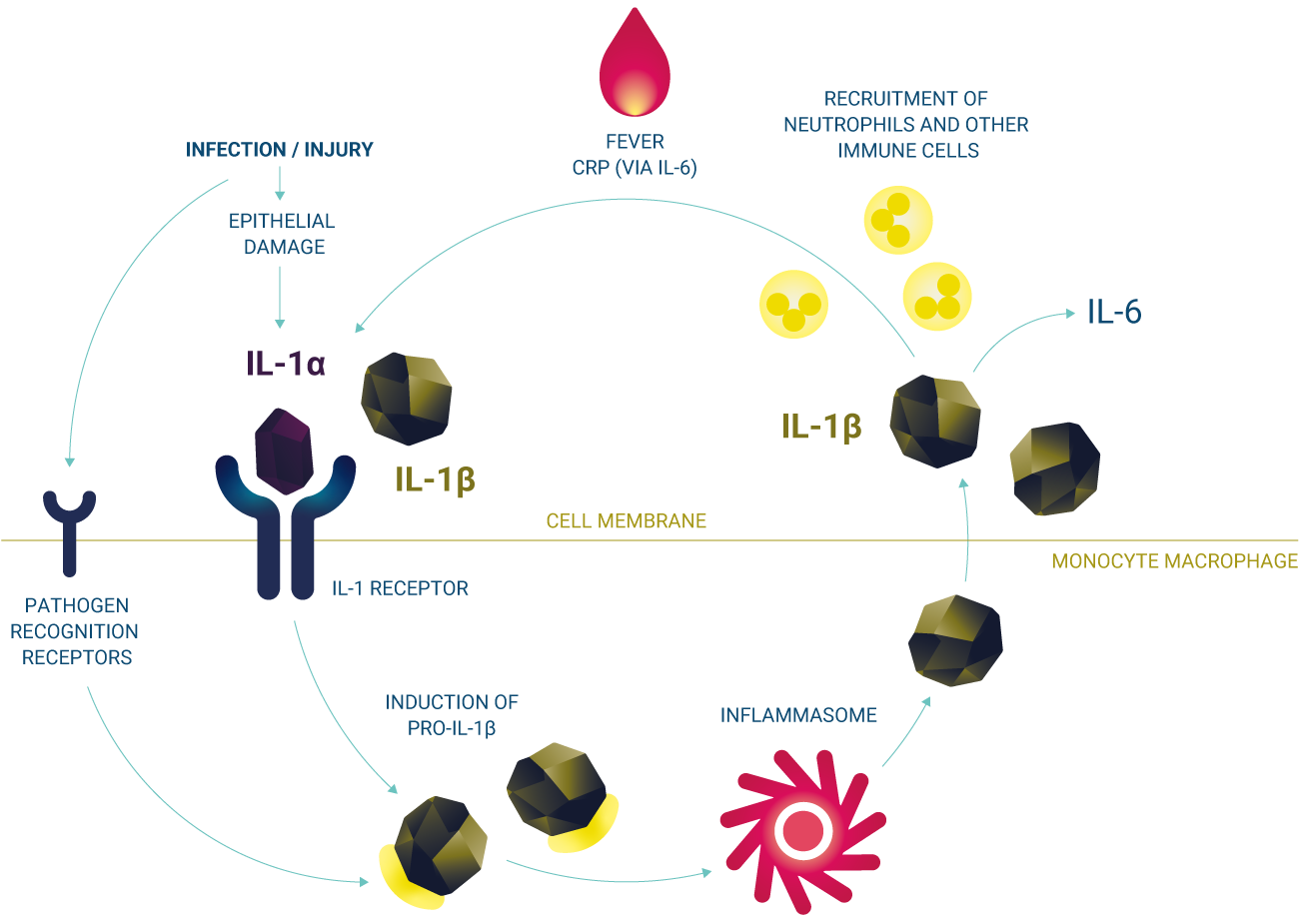
Kineret is indicated for the treatment of several diseases associated with IL-1-mediated inflammation.1
Kineret was first approved in 2001 for the treatment of Rheumatoid Arthritis (RA). Since then, its indications and use have expanded to a broad spectrum of inflammatory conditions. Kineret is included in international treatment guidelines for the management of several autoinflammatory diseases.1,2,3
Still’s disease
Kineret is indicated in adults, adolescents, children and infants aged 8 months and older with a body weight of 10 kg or above for the treatment of Still’s disease, including Systemic Juvenile Idiopathic Arthritis (SJIA) and Adult-Onset Still’s Disease (AOSD), with active systemic features of moderate to high disease activity, or in patients with continued disease activity after treatment with non-steroidal anti-inflammatory drugs (NSAIDs) or glucocorticoids.1 Kineret can be given as monotherapy or in combination with other anti-inflammatory drugs and disease-modifying antirheumatic drugs (DMARDs).1
Familial Mediterranean Fever (FMF)
Kineret is indicated for the treatment of Familial Mediterranean Fever (FMF). Kineret should be given in combination with colchicine, if appropriate.1
Rheumatoid Arthritis (RA)
Kineret is indicated in adults for the treatment of the signs and symptoms of RA in combination with methotrexate, with an inadequate response to methotrexate alone.1
Cryopyrin-Associated Periodic Syndromes (CAPS)
Kineret is indicated for the treatment of the following autoinflammatory periodic fever syndromes in adults, adolescents, children and infants aged 8 months and older with a body weight of 10 kg or above: Cryopyrin-Associated Periodic Syndromes (CAPS) Kineret is indicated for the treatment of CAPS, including: Neonatal-Onset Multisystem Inflammatory Disease (NOMID) / Chronic Infantile Neurological, Cutaneous, Articular Syndrome (CINCA) Muckle-Wells Syndrome (MWS) Familial Cold Autoinflammatory Syndrome (FCAS).1
References
-
Kineret Summary of Product Characteristics. https://www.medicines.org.uk/emc/product/559/smpc (Accessed March 2025).
-
Romano M, Arici ZS, Piskin D, Alehashemi S, Aletaha D, Barron K, et al. The 2021 EULAR/American College of Rheumatology Points to Consider for Diagnosis, Management and Monitoring of the Interleukin-1 Mediated Autoinflammatory Diseases: Cryopyrin-Associated Periodic Syndromes, Tumour Necrosis Factor Receptor-Associated Periodic Syndrome, Mevalonate Kinase Deficiency, and Deficiency of the Interleukin-1 Receptor Antagonist. Arthritis Rheumatol. 2022 Jul 1;74(7):1102–21.
-
Ozen S, Demirkaya E, Erer B, Livneh A, Ben-Chetrit E, Giancane G, et al. EULAR recommendations for the management of familial Mediterranean fever. Ann Rheum Dis. 2016 Apr;75(4):644–51.
-
EMA. Kineret: EPAR - Risk-management plan. Available from: https://www.ema.europa.eu/en/documents/rmp-summary/kineret-epar-risk-management-plan_en.pdf (Accessed March 2025).
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard or search MHRA Yellow Card in the Google Play or Apple App Store (for United Kingdom) and www.hpra.ie (for Republic of Ireland). Adverse events should also be reported to Swedish Orphan Biovitrum Ltd at [email protected] or Telephone +44 (0) 800 111 4754