Click here for United Kingdom
prescribing information
Click here for Republic of Ireland
prescribing information
Adverse Event Reporting details can be found at the bottom of this page
Guidelines
Doptelet is a guideline-recommended TPO-RA1
ICR, ASH and European JWG recommend limiting steroid use and moving onto TPO-RAs1–3
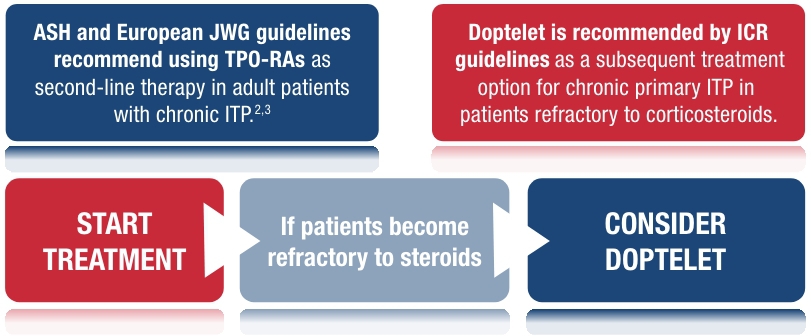
Guidelines encourage shared decision making and recommend considering patient preference,
adherence and impact on quality of life when it comes to treatment choice.1–3
Use Doptelet as your first choice for your steroid-refractory adult patients with primary chronic ITP
*Grade A recommendation, ≥1 RCT as part of a body of literature of overall good quality and consistency addressing specific recommendation; evidence level Ib, evidence obtained from ≥1 RCT.1
Doptelet is indicated for the treatment of primary chronic immune thrombocytopenia (ITP) in adult patients who are refractory to other treatments (e.g. corticosteroids, immunoglobulins).4
ASH, American Society of Hematology; ICR, International Consensus Report; ITP, immune thrombocytopenia; JWG, Joint Working Group; RCT, randomised control trial; TPO-RA, thrombopoietin receptor agonist.
References
1. Provan D et al. Blood Adv. 2019; 3(22):3780–3817. 2. Neunert CE et al. Blood Adv. 2019; 3(23):3829–3866. 3. Matzdorff A et al. Oncol Res Treat. 2018; 41 Suppl 5:1–30. 4. Doptelet EMC Summary of Product Characteristics. Available at: www.medicines.org.uk/emc/product/11837/smpc#gref Last accessed: April 2025.
Doptelet is a registered trademark of AkaRx, Inc., a Sobi company. Sobi is a trademark of Swedish Orphan Biovitrum AB (publ) © 2025 Sobi, Inc. – All rights reserved.
Adverse events should be reported. Reporting forms and information can be found at
www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App
Store (for United Kingdom) and www.hpra.ie (for Republic of Ireland). Adverse events should also be reported to Swedish Orphan Biovitrum Ltd at [email protected] or Telephone +44 (0) 800 111 4754
- Home
- Therapeutic areas
-
Products
- Alprolix (eftrenonacog alfa)
- Aspaveli®▼ (pegcetacoplan)
- Doptelet® (avatrombopag)
- Kineret® (anakinra)
- Orfadin® (nitisinone)
- Waylivra▼ (volanesorsen)
- Zynlonta®▼ (loncastuximab tesirine)
- Resources
- Events
- Contact



