This medicinal product has been authorised under a 'conditional approval' scheme. This means that further evidence on this medicinal product is awaited.
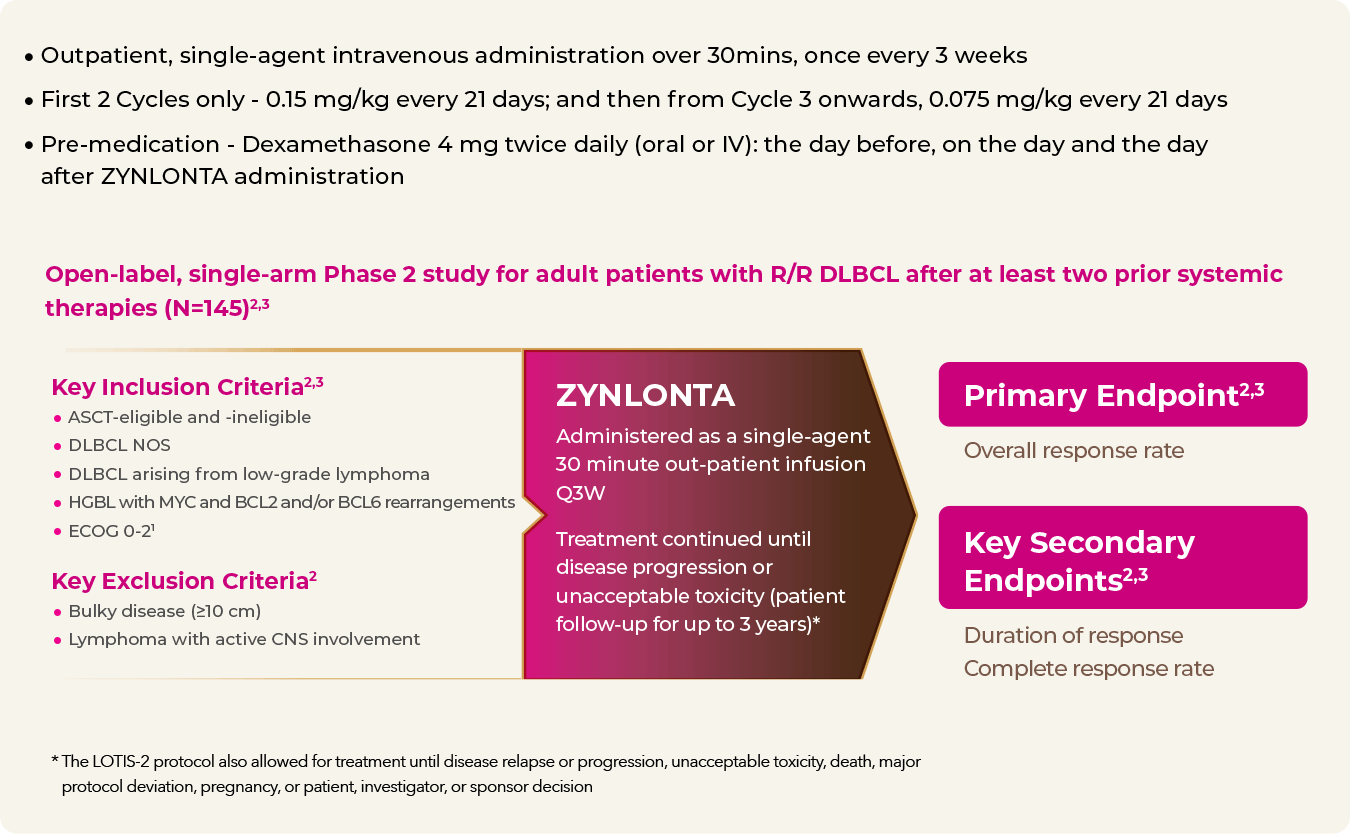
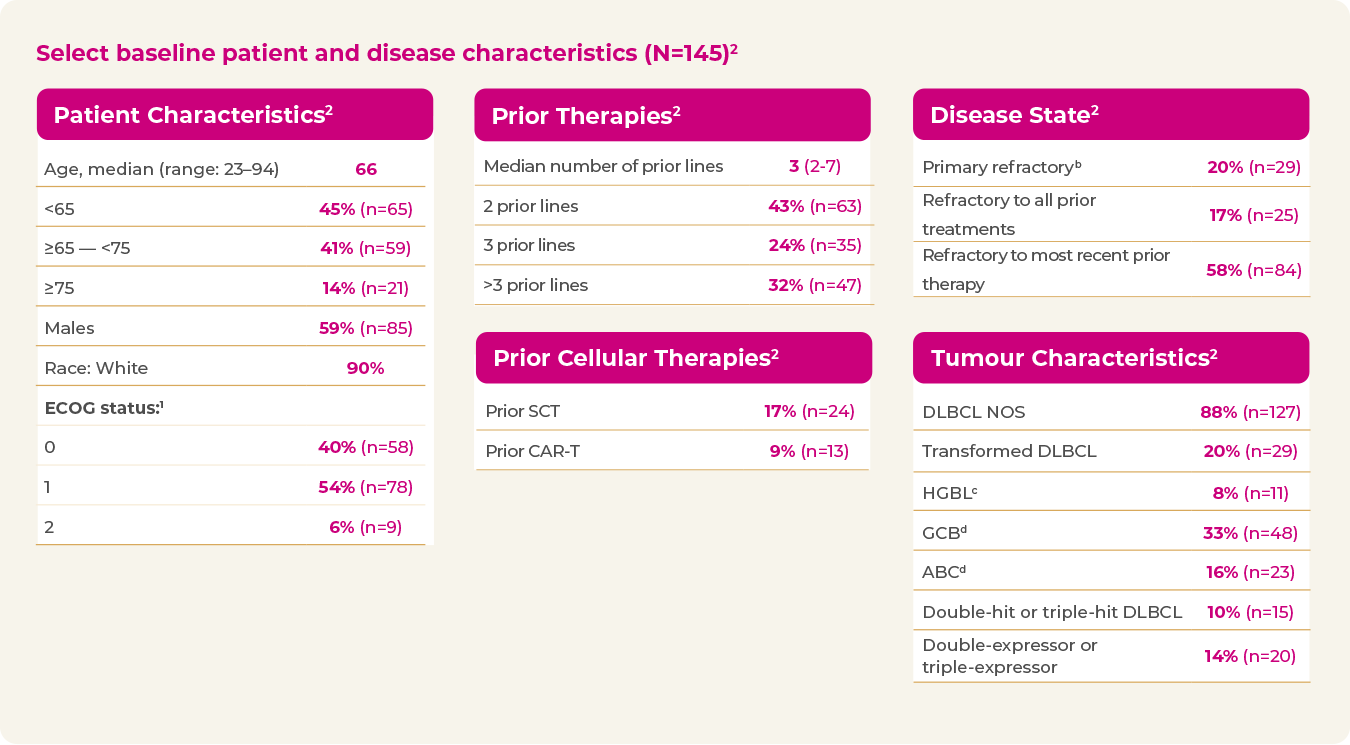
A heterogeneous and highly refractory patient population2
- 20% primary refractory
- 58% refractory to last line of treatment
- 26% received prior SCT or CAR-T

▼This medicine is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Swedish Orphan Biovitrum Ltd by email at [email protected] or by calling +44 (0) 800 111 4754.
a Of patients with a CR. Event-free defined as no progressive disease or death starting from day 1, cycle 1 of ZYNLONTA treatment.
b Updated 2-year analysis. Median follow-up: 7.8 months (range, 0.3–42.6) in the all-treated population and 35.0 months (range, 4.4–42.6) in patients with a CR.1
c Of patients with a CR. Event-free defined as no progressive disease or death starting from day 1, cycle 1 of ZYNLONTA treatment.
d Each bar represents 1 of the 36 patients with a CR in the study. Response was determined by an independent reviewer.
e Reasons for censoring patients with a CR included study discontinuation in 15 (41.7%) patients, SCT in 10 (27.8%) patients, and start of new anticancer therapy in 5 (13.9%) patients; 3 (8.3%) patients each experienced progressive disease and death.







