LOTIS-2: Primary analysis results. A pivotal trial designed to reflect patients with R/R DLBCL in clinical practice1,2
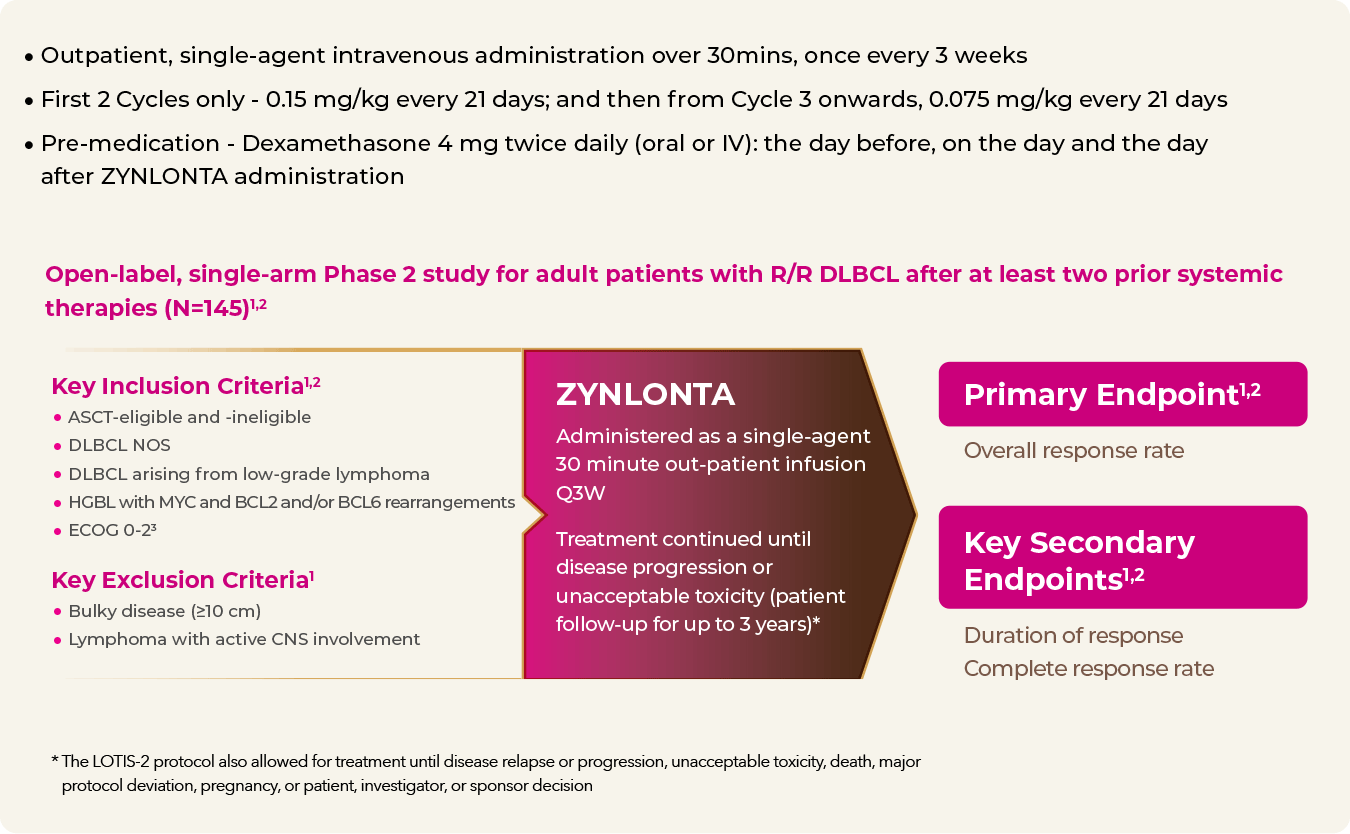
ZYNLONTA trial administration2
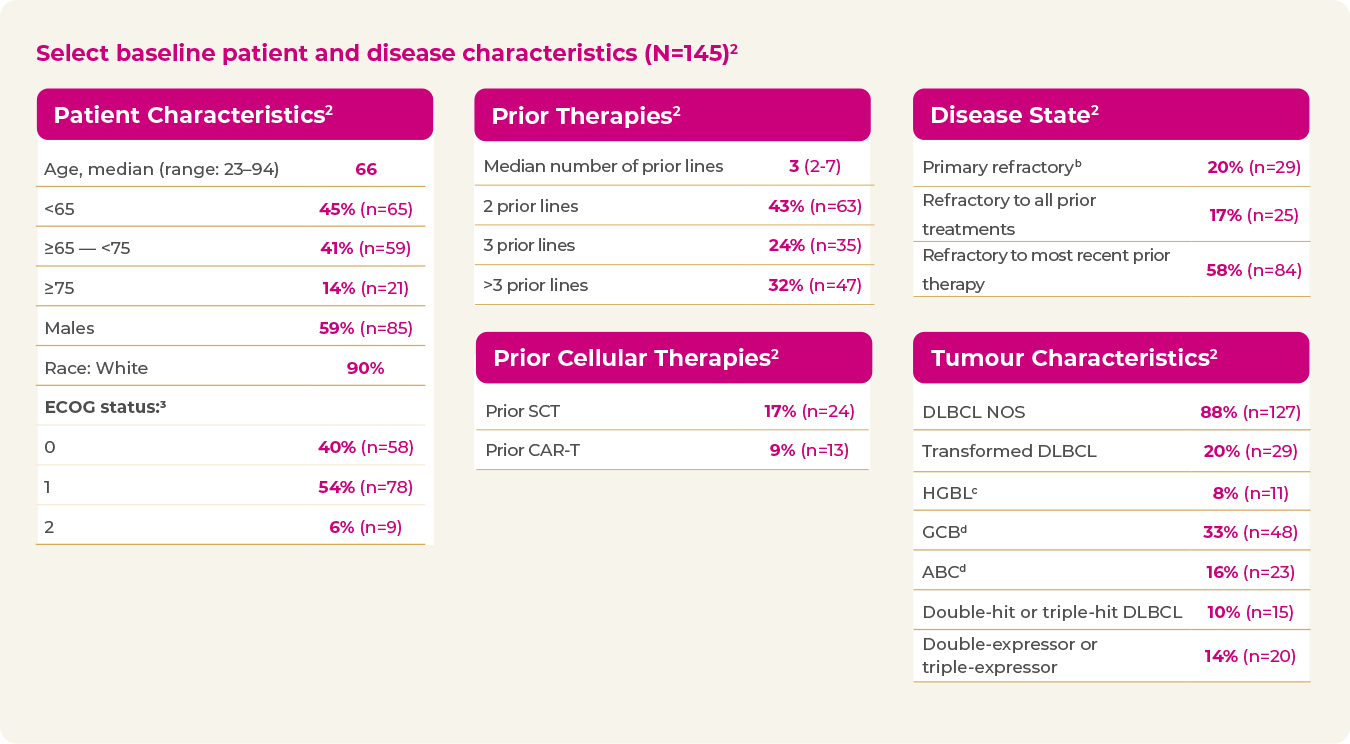
A heterogeneous and highly refractory patient population2
- 20% primary refractory
- 58% refractory to last line of treatment
- 26% received prior SCT or CAR-T
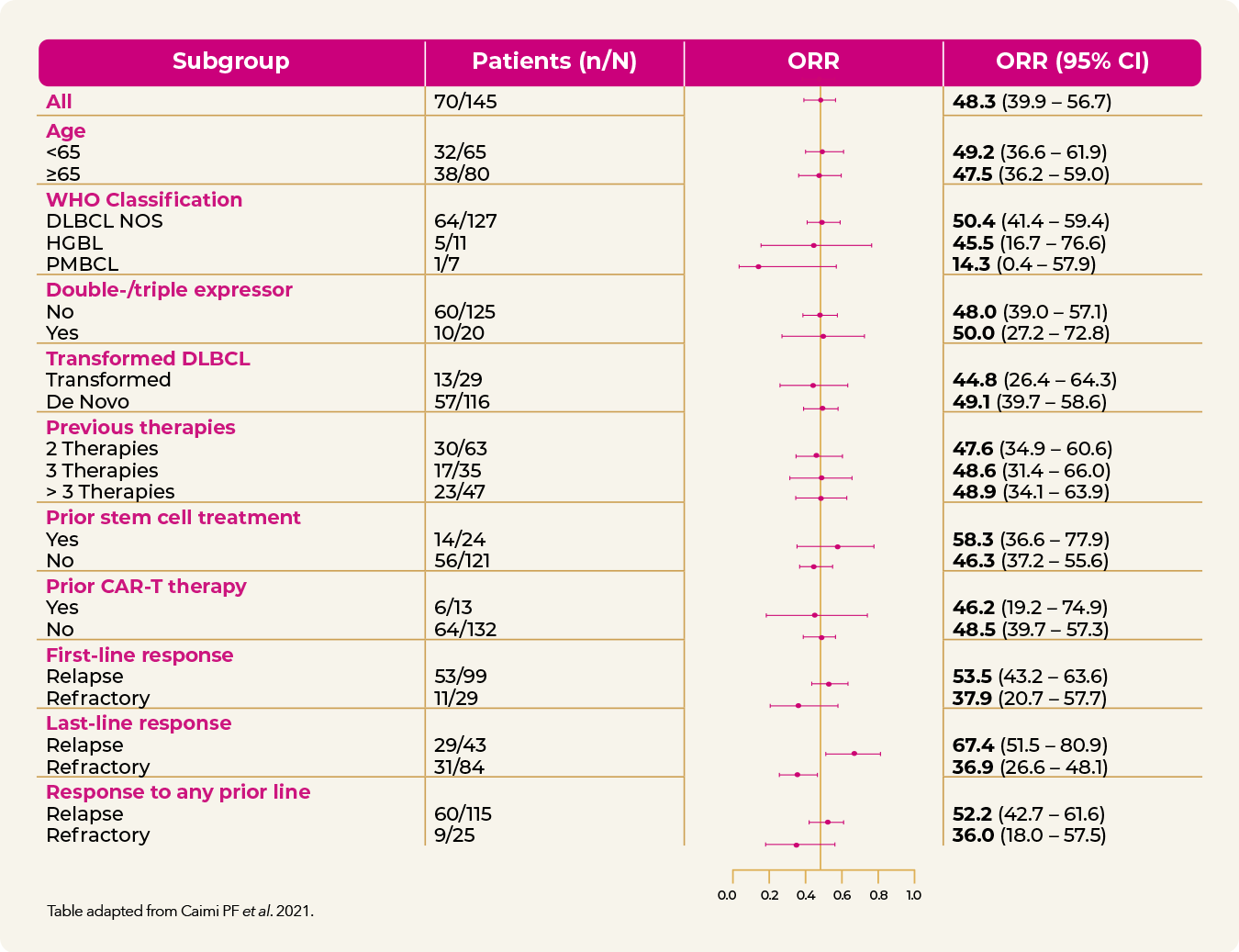
LOTIS-2: ZYNLONTA as a single agent achieves substantial responses within 1.3 months (range 1.1 – 8.1)1,e

▼This medicine is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Swedish Orphan Biovitrum Ltd by email at [email protected] or by calling +44 (0) 800 111 4754.
a The LOTIS-2 protocol also allowed for treatment until disease relapse or progression, unacceptable toxicity, death, major protocol deviation, pregnancy, or patient, investigator, or sponsor decision.
b Primary refractory defined as no response to front-line therapy.
c HGBCL with MYC and BCL2 and/or BCL6 rearrangements.
d GCB and ABC were investigator-reported without independent testing; unknown 51%.
e Median follow-up: 7.8 months (range: 0.3-31).1
Abbreviations:
Q3W: once every 3 weeks, R/R 3L+ DLBCL: relapsed/refractory third-line plus diffuse large B-cell lymphoma, NOS: not otherwise specified, ASCT: autologous stem-cell transplantation, HGBL: high-grade B-cell lymphoma, GCB: germinal centre B cell, ABC: activated B cell, SCT: stem cell transplantation, CAR-T: chimeric antigen receptor T-cell, ECOG: Eastern Cooperative Oncology Group, ORR: overall response rate, CI: confidence interval, CR: complete response rate, NE: not estimable, PMBCL: primary mediastinal large B-cell lymphoma, WHO: World Health Organisation