
Familial Chylomicronemia Syndrome (FCS)
FCS is a genetic disorder of lipid metabolism resulting in severe hypertriglyceridemia and increased risk of recurrent and potentially fatal pancreatitis.1
Patients with FCS have plasma triglyceride (TG) levels ranging from 10 to 100 times the normal value.1 Chylomicrons are formed in the intestines and transport triglycerides from the intestines to the rest of the body.2
FCS is a rare autosomal recessive disease associated with limited functionality of lipoprotein lipase (LPL) resulting in accumulation of triglycerides in the blood, carried primarily in chylomicrons (chylomicronemia).1 The accumulation of chylomicrons leads to different symptoms. The most severe consequence is increased risk of recurrent and potentially fatal acute pancreatitis.1,3
Patients affected by chylomicronemia due to lipoprotein lipase deficiency are at much greater risk of pancreatitis compared with patients with hypertriglyceridemia caused by other conditions, suggesting a direct correlation between accumulation of chylomicrons and pancreatitis.3
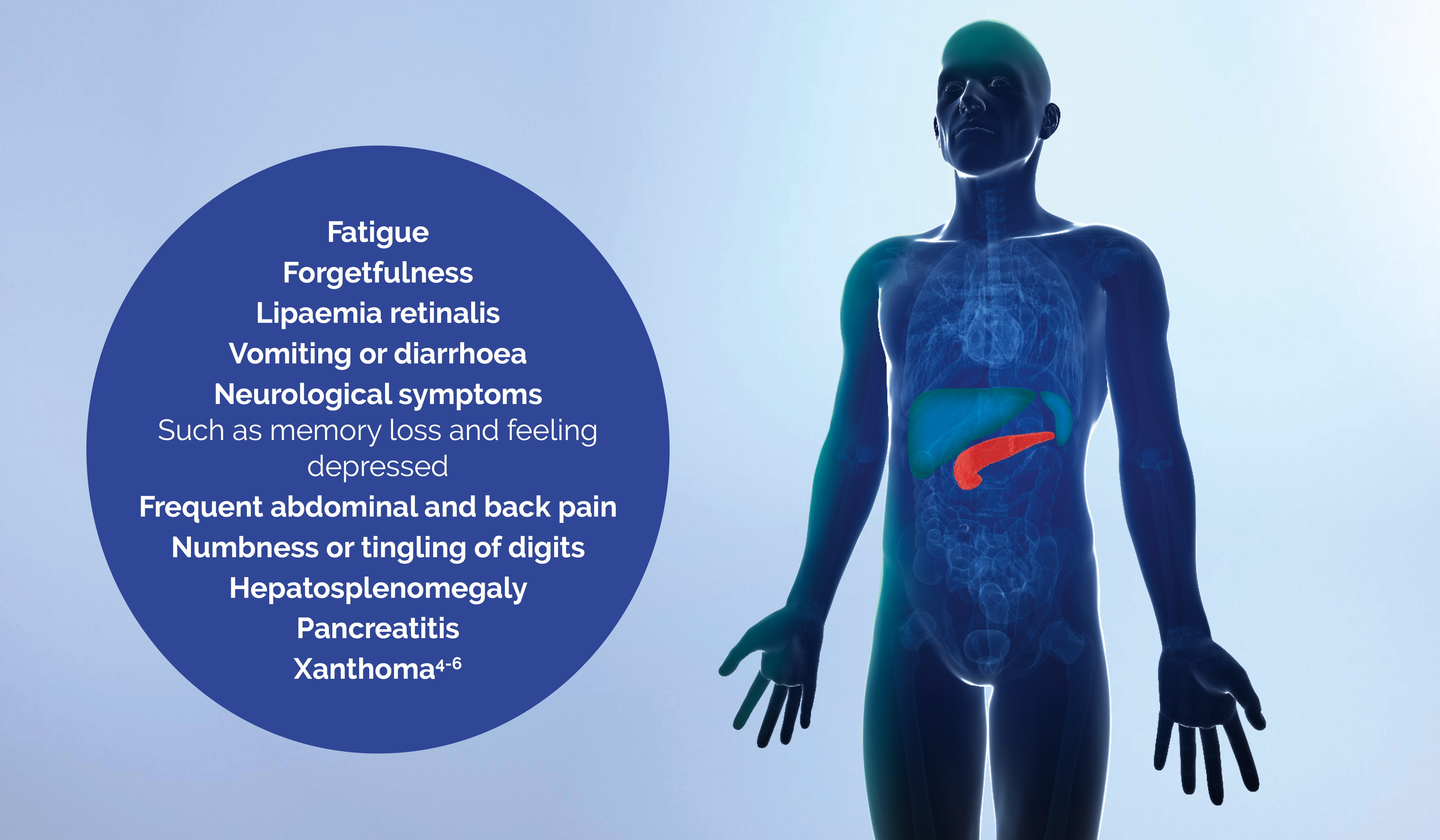
How FCS affects daily living
Patients with FCS frequently experience manifestations that have a considerable impact on their daily lives. 75% of patients have felt that their social relationships were impacted by FCS.4
FCS: familial chylomicronemia syndrome, LPL: lipoprotein lipase, TG: triglycerides
References
- Gaudet D et al. N Engl J Med 2014;371(23):2200–6.
- Feingold KR. In: Feingold KR et al. Editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK305896/ (Accessed Mar 2025).
- Stroes E et al. Atheroscler Suppl 2017;23:1–7.
- Davidson M et al. J Clin Lipidol 2018;12(4):898–907.e2.
- Davidson M et al. Expert Rev Cardiovasc Ther 2017;15(5):415–23.
- Brunzell JD, Bierman EL. Med Clin North Am 1982;66(2):455–68.
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Swedish Orphan Biovitrum Ltd at [email protected] or Telephone +44 (0) 800 111 4754


