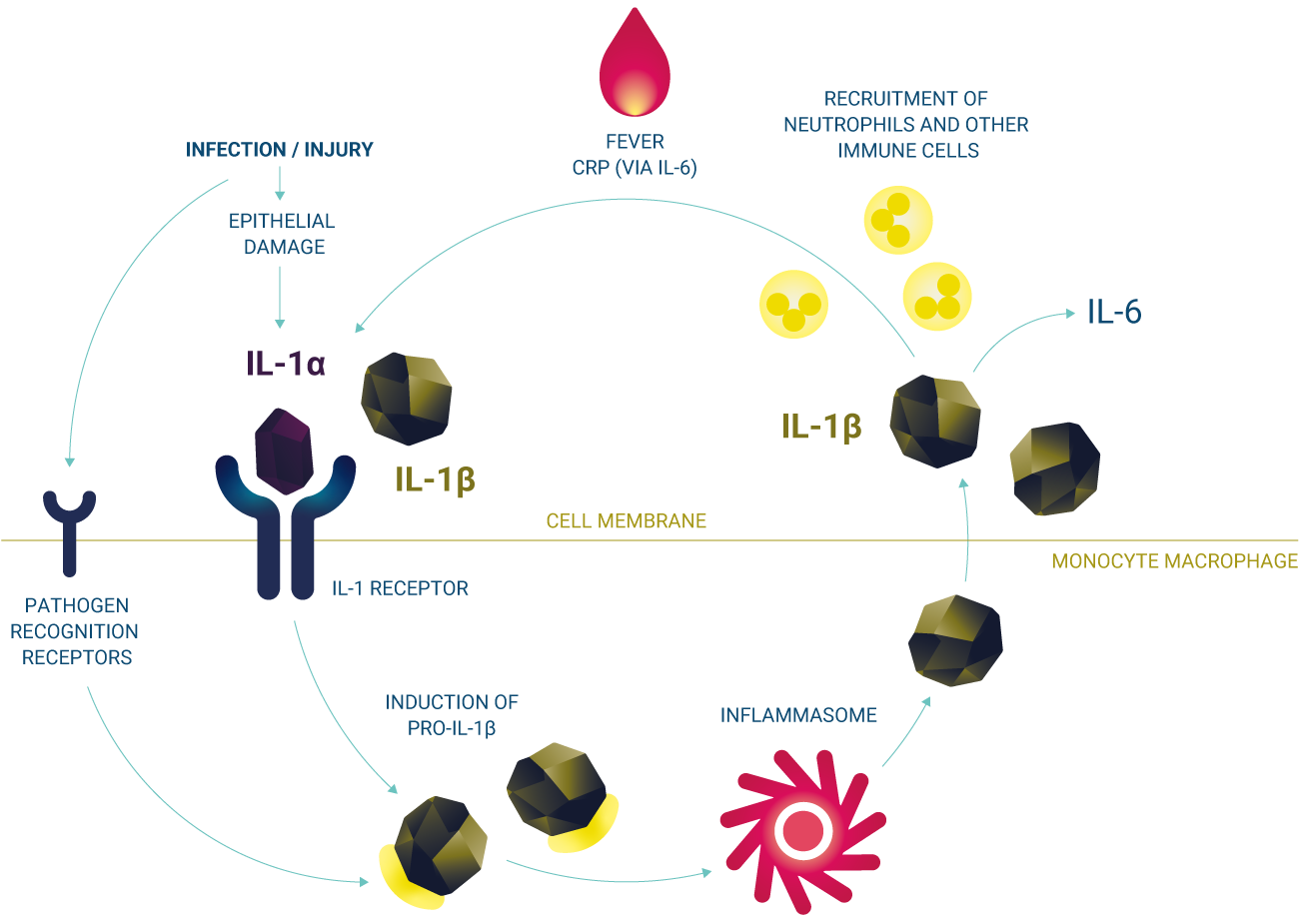
Kineret is a recombinant version of the naturally occurring IL-1R1. Kineret acts by competitively inhibiting the biological activity of IL-1α and IL-1β, preventing both isoforms from attaching and initiating downstream signalling cascades.1,2 By blocking the interaction between IL-1α/IL-1β and IL-1R1, Kineret interrupts IL-1-mediated inflammatory processes.2
The absolute bioavailability of Kineret after a 70 mg subcutaneous bolus injection in healthy subjects (n = 11) is 95%. The absorption process is the rate-limiting factor for the disappearance of Kineret from the plasma after subcutaneous injection. In subjects with RA, maximum plasma concentrations of Kineret occurred at 3 to 7 hours after subcutaneous administration of Kineret at clinically relevant doses (1 to 2 mg/kg; n = 18). The plasma concentration decreased with no discernible distribution phase and the terminal half-life ranged from 4 to 6 hours.1
How is Kineret administered and supplied?
- Kineret is given in a pre-filled syringe containing 100 mg of anakinra per 0.67 ml (150 mg/ml).1
- Kineret is given as a subcutaneous injection.1
- Dosage is usually once daily, but varies depending on indication. In children, the dose is determined depending on body weight. Please refer to Summary of Product Characteristics (SmPC) for further information.1
How is Kineret stored?
- Kineret should be stored in a refrigerator between 2°C and 8°C (36°F and 46°F).1
- Kineret should not be frozen or shaken.1
- Kineret should be kept in its original carton and away from light.1
ACR20, American College of Rheumatology response criteria 20; CRP, C-reactive protein; IL-1, interleukin-1; IL-1R, IL-1 receptor type I; IL-1α/IL-1β, Interleukin-1 alpha/Interleukin-1 beta; RA, rheumatoid arthritis; SmPC, Summary of Product Charateristics.
References
- Kineret Summary of Product Characteristics. https://www.medicines.org.uk/emc/product/559/smpc (Accessed March 2025).
- Cavalli G, Dinarello CA. Anakinra therapy for non-cancer inflammatory diseases. Front Pharmacol. 2018;9:1157.
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard or search MHRA Yellow Card in the Google Play or Apple App Store (for United Kingdom) and www.hpra.ie (for Republic of Ireland). Adverse events should also be reported to Swedish Orphan Biovitrum Ltd at [email protected] or Telephone +44 (0) 800 111 4754