Well-established safety profile1–5
With ELOCTA, you can offer patients a treatment with a well-established safety profile, supported by up to 6 years of cumulative clinical trial experience and >8 years of real-world experience.1–5
In A-LONG, Kids A-LONG and the ASPIRE extension study, there were:1–5

ELOCTA was well tolerated in previously untreated patients6
In PUPs A-LONG:6
• Overall inhibitor development was in the expected range (31.1%)
- 79.6% of enrolled patients had high-risk mutations for inhibitor development
• Cumulative incidence of high-titre inhibitors was lower than in large randomised and real-world studies (16.1% in PUPs A-LONG vs 22.4% in PedNet and 28.4% in SIPPET)
Adverse reactions reported in clinical trials of ELOCTA1
The frequencies of adverse reactions in the table below were observed in a total of 276 patients with severe haemophilia A in Phase 3 clinical studies and an extension study with a duration of up to four years. The total number of exposure days was 80,848 with a median of 294 (range 1–735) exposure days per patient.
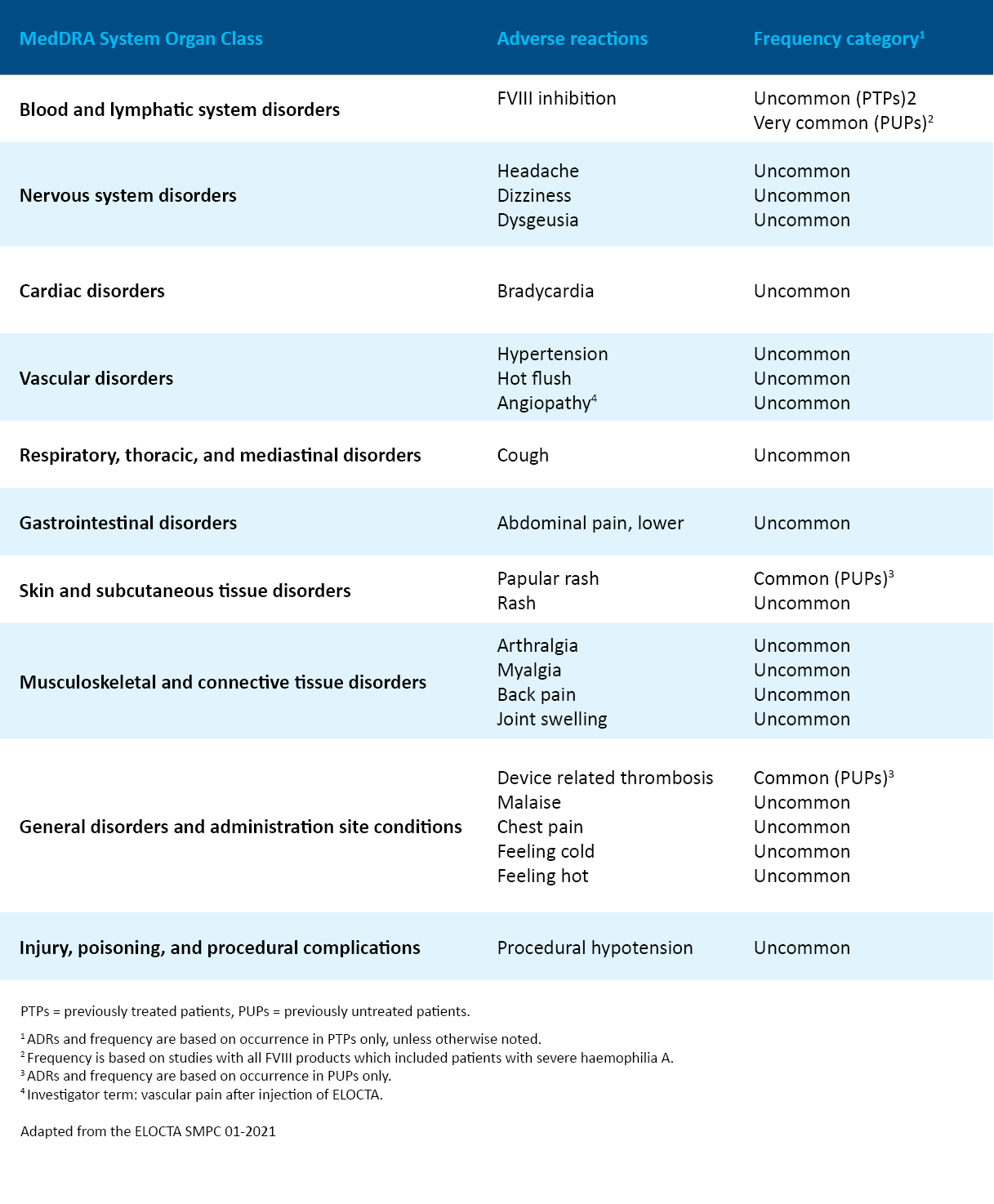
*Patients with a prior history of, or currently detectable inhibitor were excluded from the trials. Inhibitor development has been observed in clinical practice (as with other approved FVIII products).
†Patients with a history of hypersensitivity associated with any FVIII or intravenous immunoglobulin administration were excluded from the trials.
‡Frequencies have been evaluated according to the following convention: very common (≥1/10); common (≥1/100 to <1/10); uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1,000); very rare (<1/10,000), not known (cannot be estimated from the available data).
§Frequency is based on studies with all FVIII products which included patients with severe haemophilia A.
¶Investigator term: vascular pain after injection of ELOCTA.
FVIII, factor VIII; PedNet, Paediatric Network; PTP, previously treated patient; PUP, previously untreated patient; SIPPET, Survey of Inhibitors in Plasma-Product Exposed Toddlers.
References
1. ELOCTA SmPC 01/2021. 2. Nolan B, et al. Haemophilia 2020;26(3):494–502. 3. Mahlangu J, et al. Blood 2014;123(3):317–325. 4. Young G, et al. J Thromb Haemost 2015;13(6):967–977. 5. Konkle B, et al. Res Pract Thromb Haemost. 2023;7:e102180. 6. Königs C. et al. Blood. 2022;139(26):3699-3707.


