Proven protection1–4
The efficacy and safety profile of ELOCTA is supported by a robust clinical trial programme.1–4
Learn more about the ELOCTA clinical trials below.
A-LONG: Data in adults and adolescents1
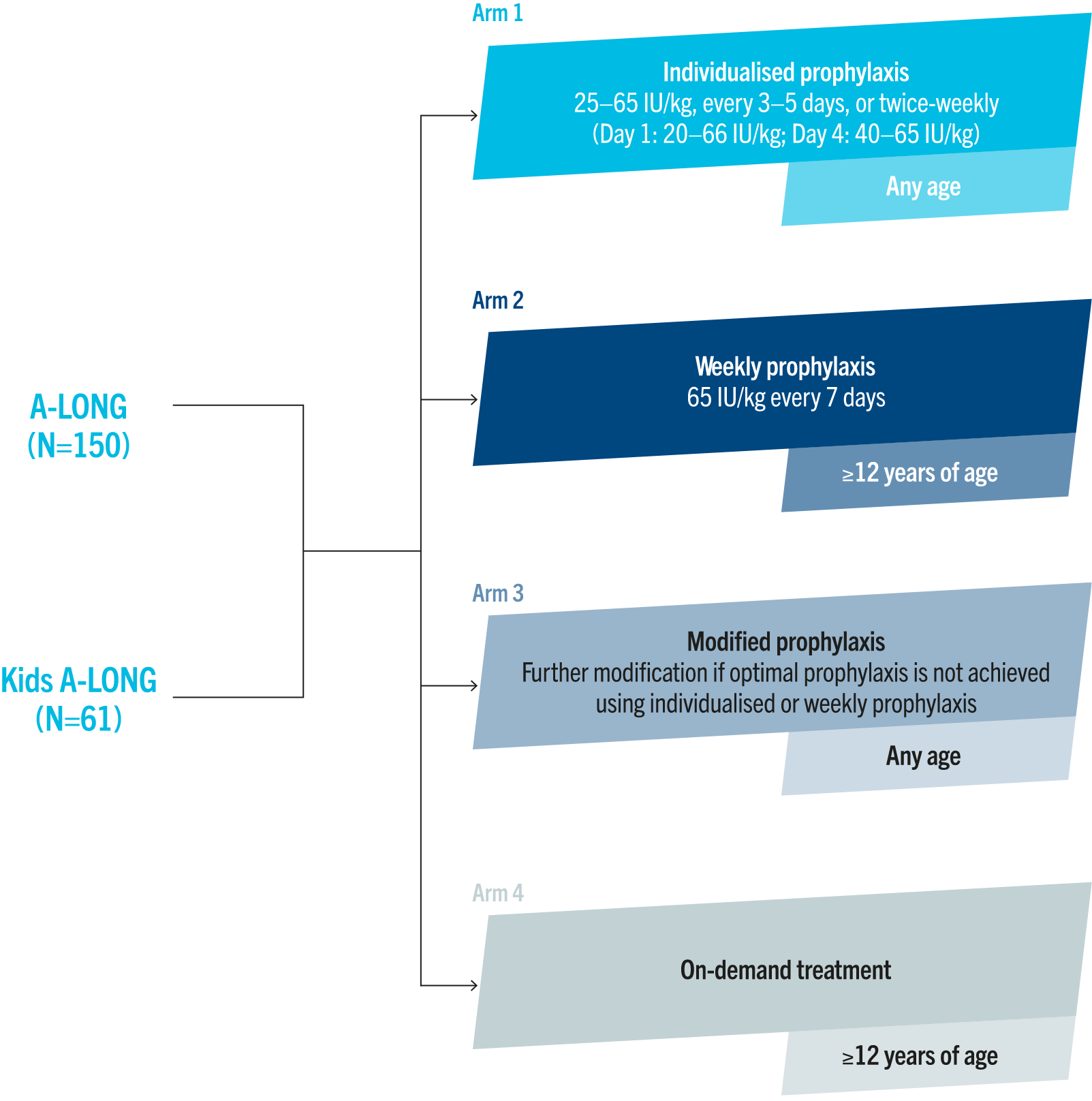
A-LONG was an open-label, multinational, Phase 3 trial evaluating the safety, efficacy and PK properties of ELOCTA in 165 previously treated males with severe haemophilia A.1
The primary endpoints were:1
• ABR in individualised prophylaxis vs on demand
• assessment of FVIII activity based on PK properties
• inhibitor development
• adverse events
A-LONG trial design1
Adapted from Mahlangu J, et al. Blood 2014.
Kids A-LONG and PUPs A-LONG: Data in children and previously untreated patients2,3
Two clinical trials were conducted to evaluate the efficacy and safety profile of ELOCTA in children. Previously treated patients were enrolled in the Kids A-LONG trial and previously untreated patients were enrolled in the PUPs A-LONG trial.2,3
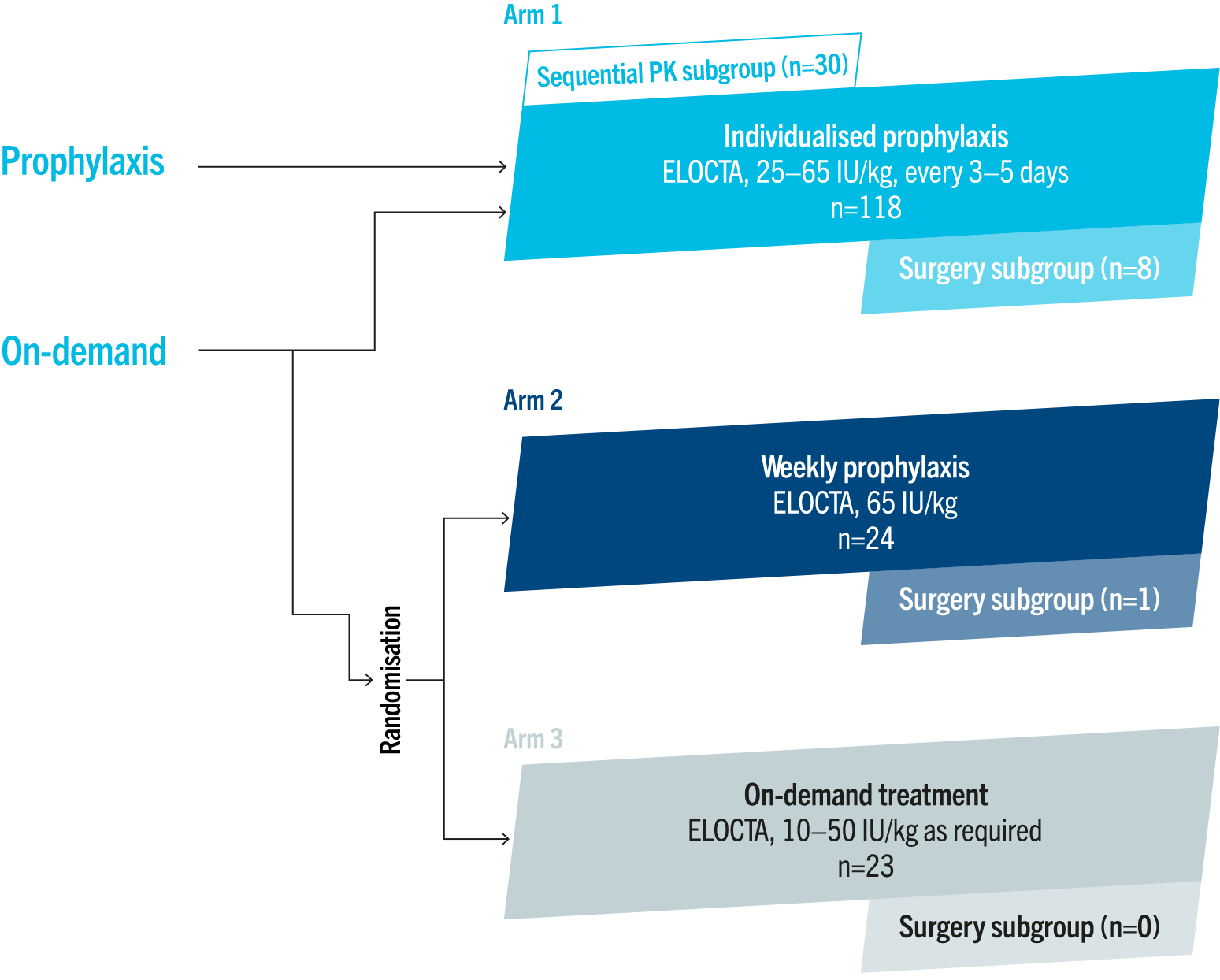
Kids A-LONG was an open-label, multinational, Phase 3 trial evaluating the safety, efficacy and PK properties of ELOCTA in 71 previously treated children with severe haemophilia A.2
The primary endpoint was:4
• inhibitor development
Secondary endpoints included:2,4
• assessment of FVIII activity based on PK properties
• ABRs
• resolution of bleeds
Kids A-LONG trial design2
Adapted from Young G, et al. J Thromb Haemost 2015.
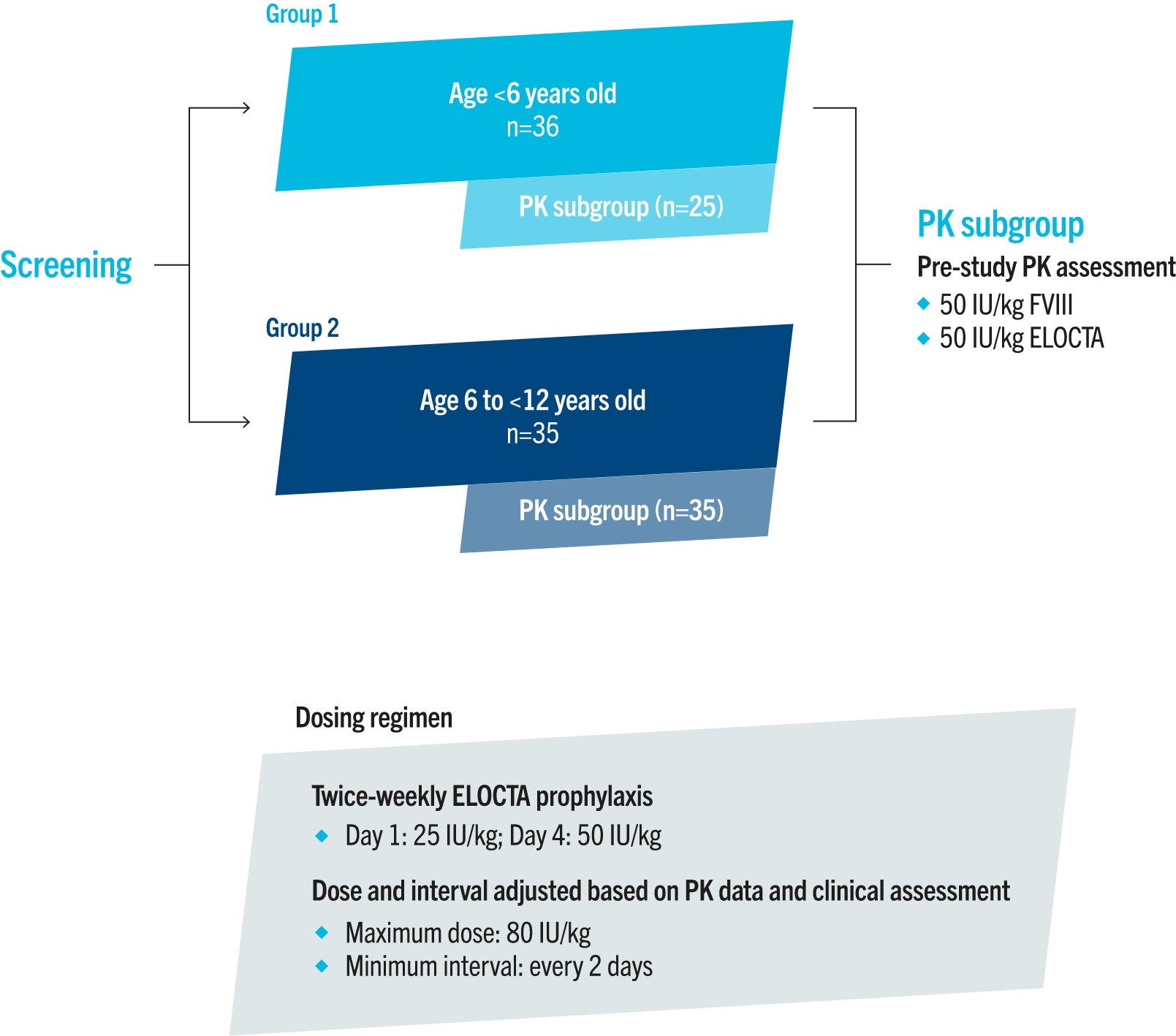
PUPs A-LONG was a Phase 3, open-label, single-arm, multicentre study evaluating the safety profile and efficacy of ELOCTA in 103 previously untreated children with severe haemophilia A.3
The primary endpoint was:3
• inhibitor development
Secondary endpoints included:3
• cumulative incidence of inhibitors
• ABRs
• annualised consumption of ELOCTA
PUPs A-LONG trial design3

Adapted from Königs C, et al. Blood.2022;139(26):3699-3707.
ASPIRE: Long-term outcomes in adults, adolescents and children4
ASPIRE was a Phase 3, open-label, multicentre extension trial evaluating the long-term safety profile and efficacy of ELOCTA in patients who completed A-LONG and Kids A-LONG.4
The primary endpoint was:4
• inhibitor development
Secondary endpoints included:4
• ABRs
• bleed resolution (assessed by both physicians and patients)
• weekly prophylactic dosage of ELOCTA
• annualised consumption of ELOCTA
ASPIRE trial design4