Durable response within 8 days
Doptelet helps get patients to the ≥50 x 109/L target within days, and keeps them there for months*1,2
The efficacy and safety of Doptelet in adult patients with chronic ITP was evaluated in a Phase 3, multicentre, randomised, double-blind, parallel-group, placebo-controlled study.1
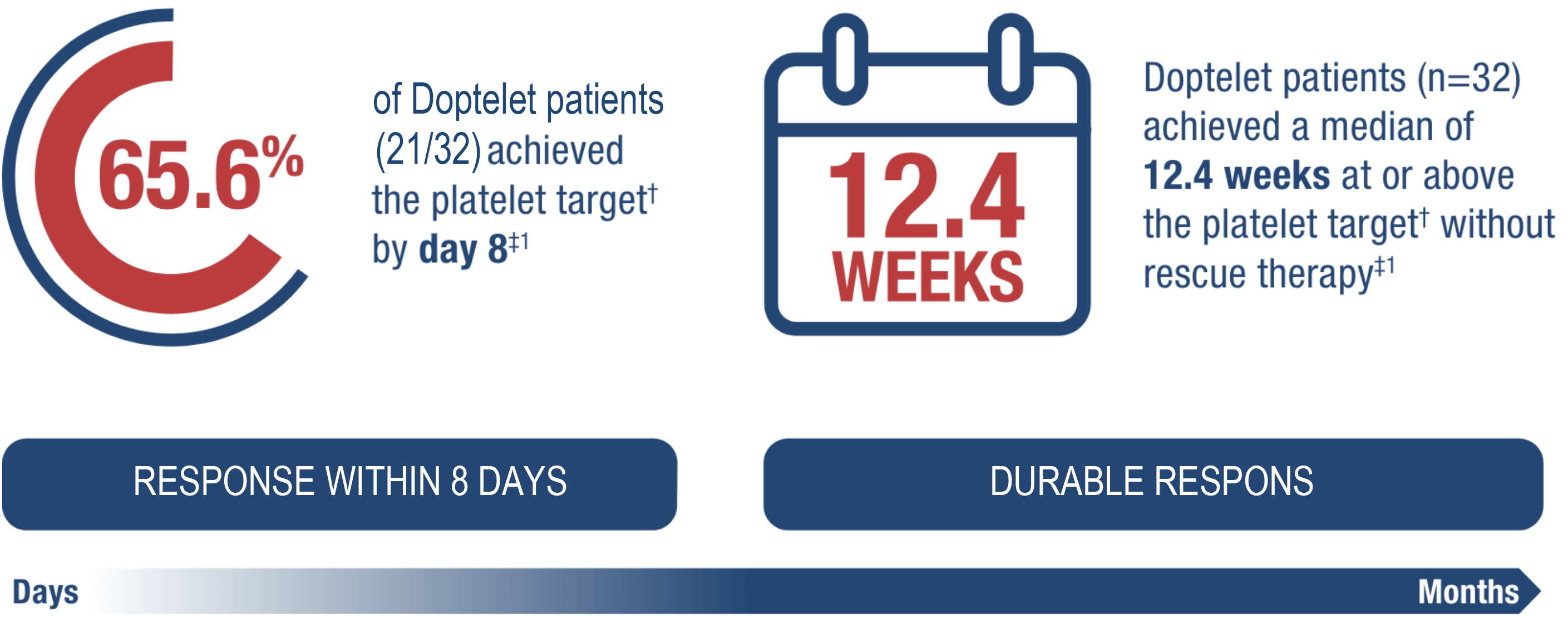
*Significantly more Doptelet patients achieved response by month 6 (87.5% vs. 5.9% placebo; P<0.0001).2
†Defined as a platelet count ≥50 x 109/L in the absence of rescue therapy.
‡Vs 0.0 with placebo (P<0.0001).1
Primary endpoint: Cumulative number of weeks of platelet response, defined as platelet count ≥50 x 109/L in the absence of rescue therapy over 6 months of once-daily treatment.1
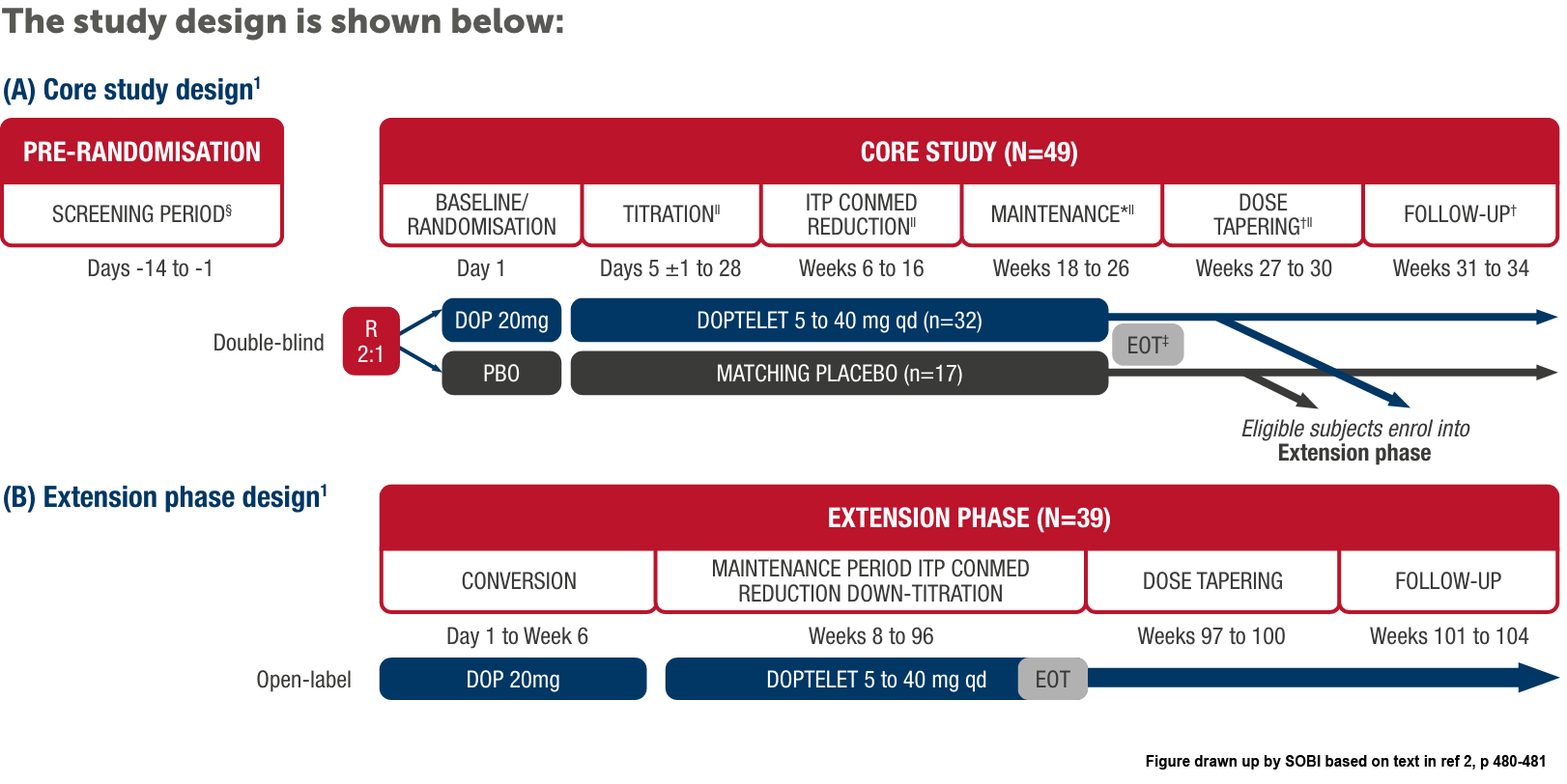
*At EOT visit (visit 22), patients could enter the extension phase. Patients not continuing entered the dose-tapering and follow-up phase.1
†For patients who did not enter the extension phase.1
‡Optional entry into the open-label extension phase.1
§The screening visit and day 1 baseline/randomisation visit platelet counts were averaged to obtain the baseline platelet count value.1
IIPatients who discontinued early who met the criteria for a lack of treatment effect may have moved directly into the open-label extension phase.1
Doptelet is indicated for the treatment of primary chronic immune thrombocytopenia (ITP) in adult patients who are refractory to other treatments (e.g. corticosteroids, immunoglobulins).3
Doptelet is indicated for the treatment of severe thrombocytopenia in adult patients with chronic liver disease who are scheduled to undergo an invasive procedure.3
ITP, immune thrombocytopenia; CONMED, concomitant medication; DOP, Doptelet; EOT, end-of-treatment; ITP, immune thrombocytopenia; qd, once daily; R, randomisation


