Aspaveli is indicated as monotherapy in the treatment of adult patients with paroxysmal nocturnal haemoglobinuria (PNH) who have haemolytic anaemia.1
Patient burden
C5i-treated patients may experience impaired QoL and absence from everyday life.4
Up to 85% of patients receiving C5i treatment do not achieve normal-like Hb levels:‡5
In survey studies, C5i-treated patients reported experiencing ongoing anaemia:4,6
- 86% (N=63) of patients in France, Germany and the UK
- 84% (N=96) of patients in the US

In patients in France, Germany and the UK:6
- 73% (n=52/71) experienced fatigue at diagnosis
- 63% (n=45/71) experienced fatigue despite C5i treatment
In C5i-treated patients in the US:4
- 79% (n=122) experienced fatigue
Patients experienced impairment in QoL across multiple EORTC QLQ-C30 categories, including:
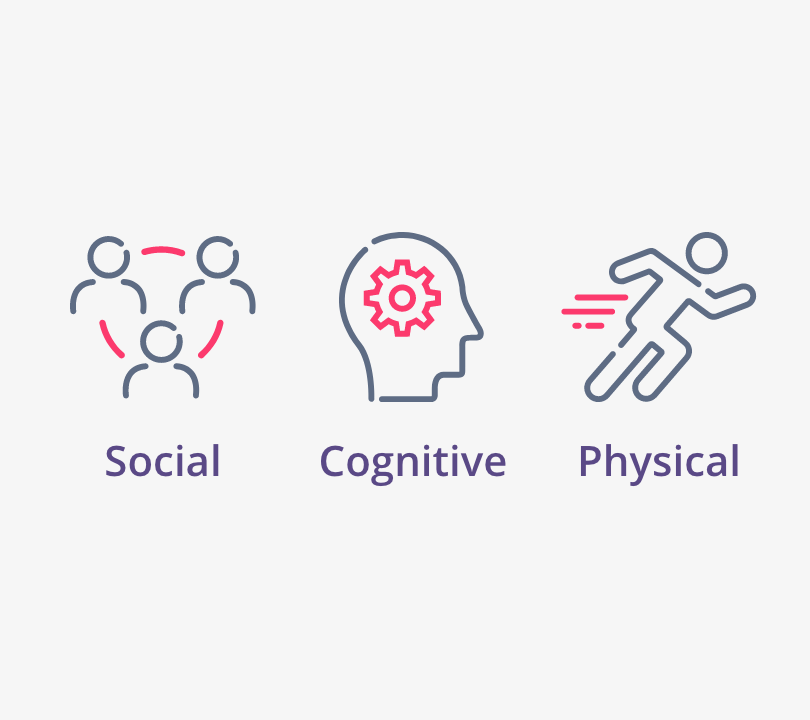
88% (n=107/122) experienced limitations in their daily activities
47% (n=25/53) experienced missing work within the previous 7 days

35% (n=19/54) of patients required ≥1 transfusion in the previous 12 months
‡Based on HCP experience, up to 85% of patients receiving eculizumab did not achieve a ‘major’ or ‘complete’ response, defined as normal-like Hb levels (≥12.0 g/dL in women, ≥13.0 g/dL in men) without requiring transfusions.5
References
1. Hillmen P et al. N Engl J Med. 2021;384:10281037. 2. Peffault de Latour R et al. Lancet Haematol. 2022;9:e648–659. 3. Aspaveli (pegcetacoplan) Summary of Product Characteristics 08/2024. 4. Dingli D et al. Ann Hematol. 2022;101(2):251–263. 5. Risitano AM et al. BJH. 2022;196:288–303. 6. Panse J et al. Eur J Haematol. 2022;109(4):351–363.