Aspaveli is indicated as monotherapy in the treatment of adult patients with paroxysmal nocturnal haemoglobinuria (PNH) who have haemolytic anaemia.1
The PEGASUS study
A Phase 3 study of Aspaveli vs eculizumab.1,3
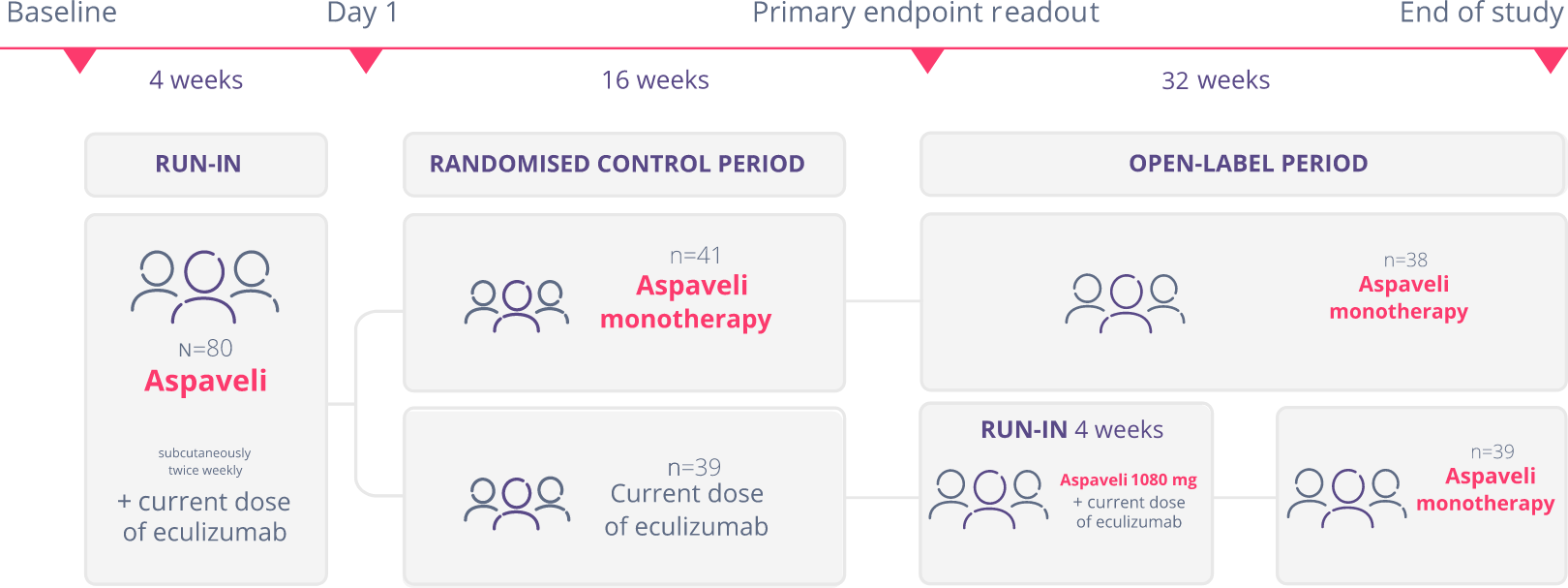
The PEGASUS study (N=80) was a Phase 3, randomised study of Aspaveli vs eculizumab, with a 16-week
active comparator controlled period, followed by a 32-week open-label period. Patients studied had PNH and Hb levels <10.5 g/dL despite treatment with eculizumab for ≥3 months.1,3
Adapted from Hillmen P et al. N Engl J Med. 2021 and Peffault de Latour R et al. Lancet Haematol. 2022.
A total of 38 patients in the Aspaveli group and 39 patients in the eculizumab group completed the 16-week randomised control period. Patients then entered a 32-week open-label period and received monotherapy with Aspaveli. Patients who received eculizumab during the 16-week randomised, controlled period continued to receive eculizumab in addition to Aspaveli for the first 4 weeks of the open-label period before starting monotherapy with Aspaveli.1,3
Primary endpoint:1
- Mean change in Hb levels from baseline to Week 16 vs eculizumab
Key secondary endpoints:1
- Proportion of patients achieving freedom from transfusions, mean change in ARC, LDH, and FACIT-Fatigue score from baseline to Week 16 vs eculizumab
Change in Hb levels
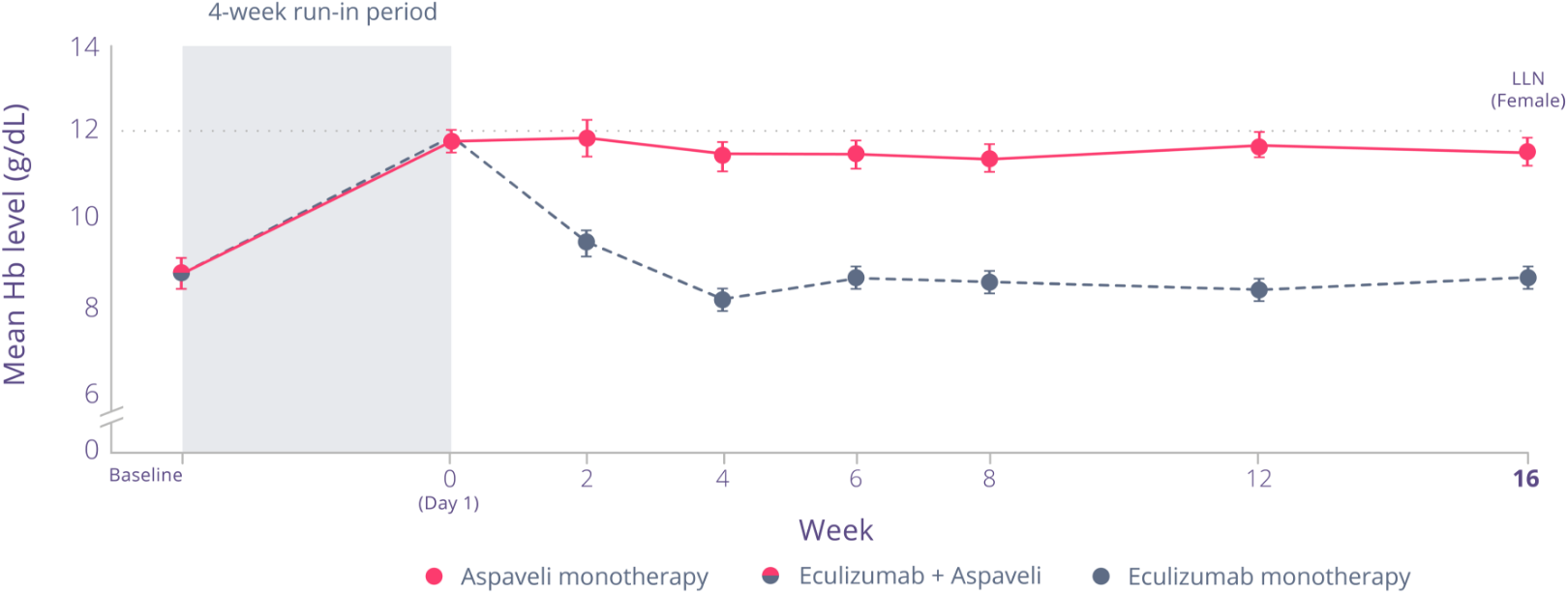
Aspaveli provides a superior increase in Hb vs eculizumab in patients with anaemia despite C5i treatment.*1
Week 16
Aspaveli was superior to eculizumab in improving Hb levels from baseline to Week 16 (P<0.001).1
Adapted from Hillmen P et al. N Engl J Med. 2021.

between treatment with Aspaveli
and eculizumab, based on each treatment’s adjusted mean change from baseline (P<0.001) (95% CI, 2.33 to 5.34)1
Week 48
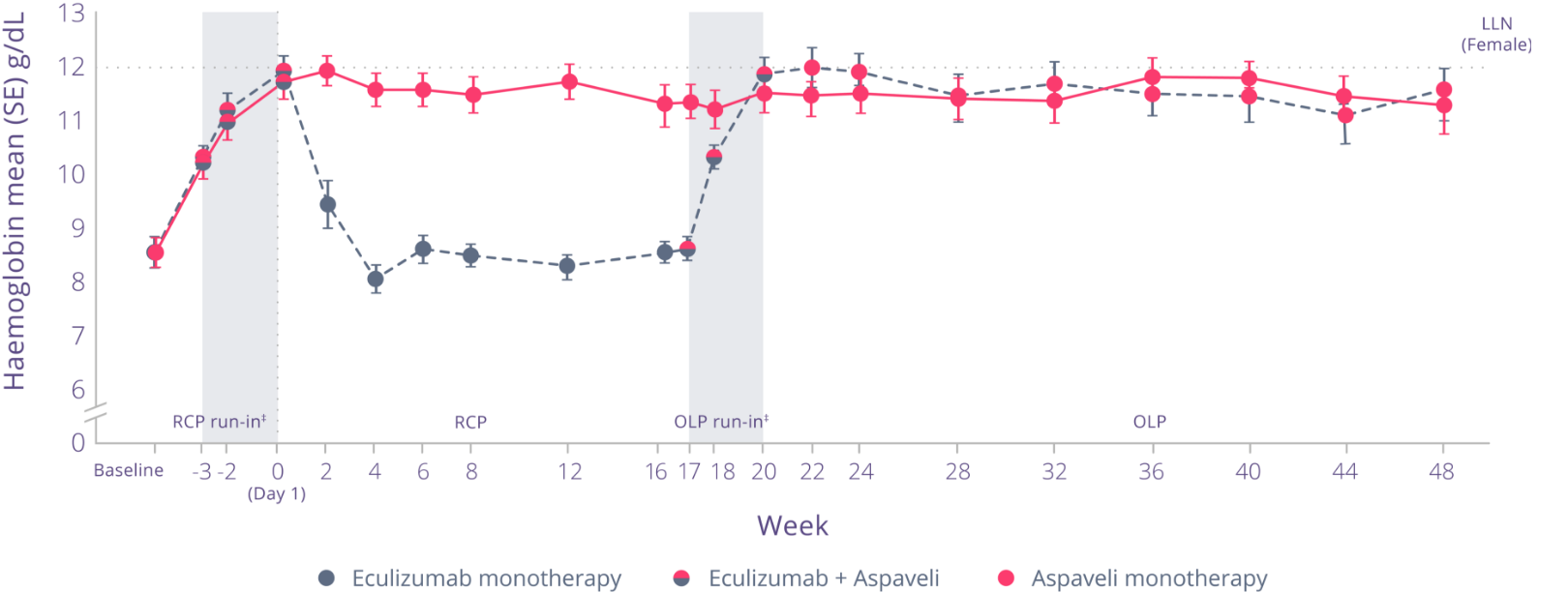
Through 48 weeks of treatment, patients who continued to receive Aspaveli achieved sustained improvements in Hb levels.*3
Adapted from Peffault de Latour R et al. Lancet Haematol. 2022.
Through Week 48:3
- Increased Hb levels were maintained in patients who continued to receive Aspaveli
- Patients who switched from eculizumab to Aspaveli at Week 16 achieved significant improvements in Hb levels (P<0.0001)
Treatment effect estimates from a mixed model are shown. The mixed model contained the categorical effects of treatment, visit, treatment by visit interaction, stratification factors (transfusion history and platelet count at screening) and the continuous covariate of baseline value.1
Primary endpoint analysis of the PEGASUS data is censored for transfusion. Transfusions could confound the results, so data after the first transfusion for all patients were not included in the primary analysis. Post-transfusion data were omitted once they had a transfusion and their data were modelled out for the remainder of the 16-week randomised control period.1
Haematologic parameters
Aspaveli provides improvements in key haematologic parameters of ongoing haemolysis.*1,3
More patients achieved normalisation of key indicators of ongoing haemolysis with
Aspaveli vs eculizumab at Week 16.*1
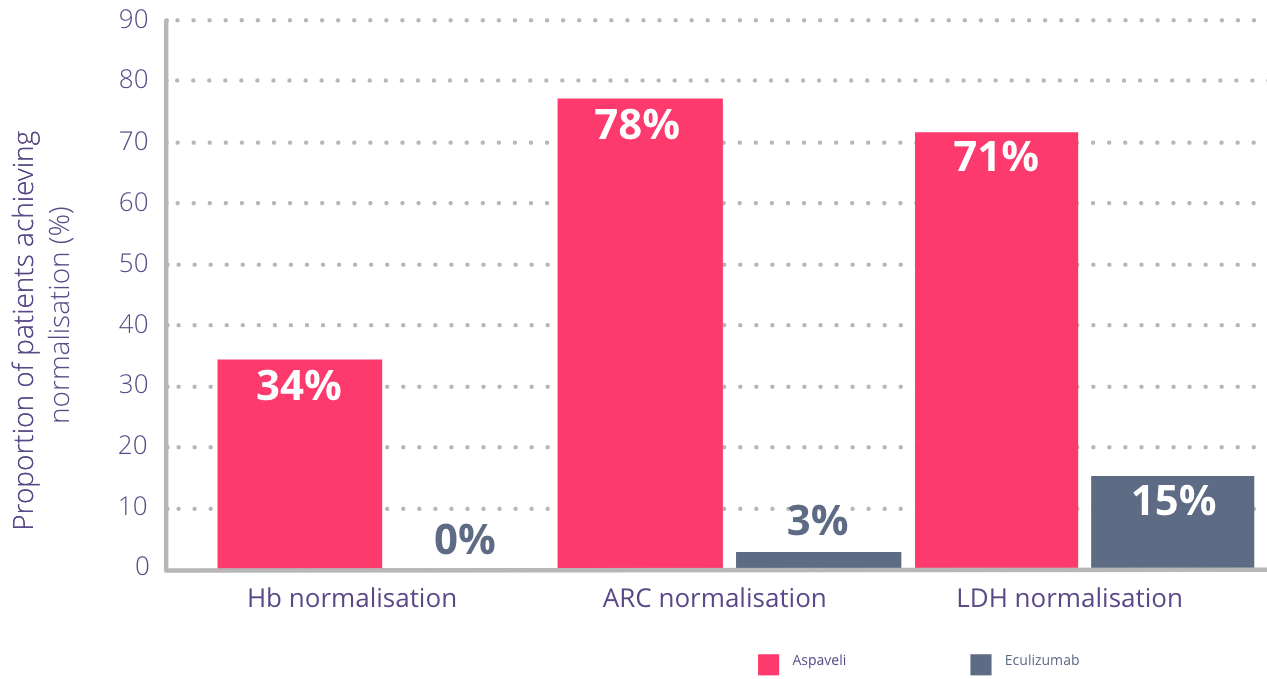
Adapted from Hillmen P et al. N Engl J Med. 2021.
Normalisation ranges are defined as follows:4
- Haemoglobin: females ≥12–16 g/dL, males ≥13.6–18 g/dL
- Reticulocytes: 30–120 x109 cells/L
- LDH: 113–226 U/L
See the impact on ARC levels
In PEGASUS, at Week 16, mean change in ARC met non-inferiority:1
- ARC (x109 cells/L) (SE) mean change from baseline was: -136 (6.5) with Aspaveli vs +28 (11.9) with eculizumab (total mean difference: 164)
With Aspaveli, improvements in ARC levels were sustained through Week 48.*3
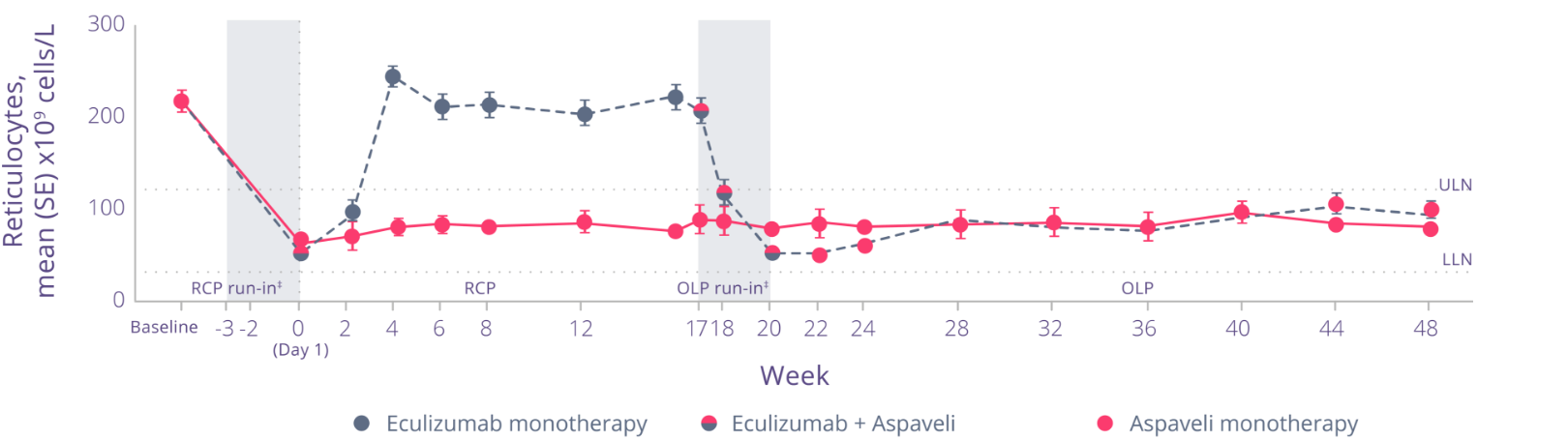
Adapted from Peffault de Latour R et al. Lancet Haematol. 2022.
- Through Week 48, decreased ARC levels were maintained in patients who continued to receive Aspaveli3
- Patients who switched from eculizumab to Aspaveli at Week 16 achieved significantly reduced ARC levels at Week 48 (P<0.0001)3
See the impact on LDH levels
In PEGASUS, at Week 16, mean change in LDH levels (U/L) was:*1
- -15 (42.7) with Aspaveli vs ‐10 (71.0) with eculizumab (total mean difference: 5)
Non-inferiority was not met in change from baseline in LDH.
With Aspaveli, improvements in LDH levels were sustained through Week 48.*3
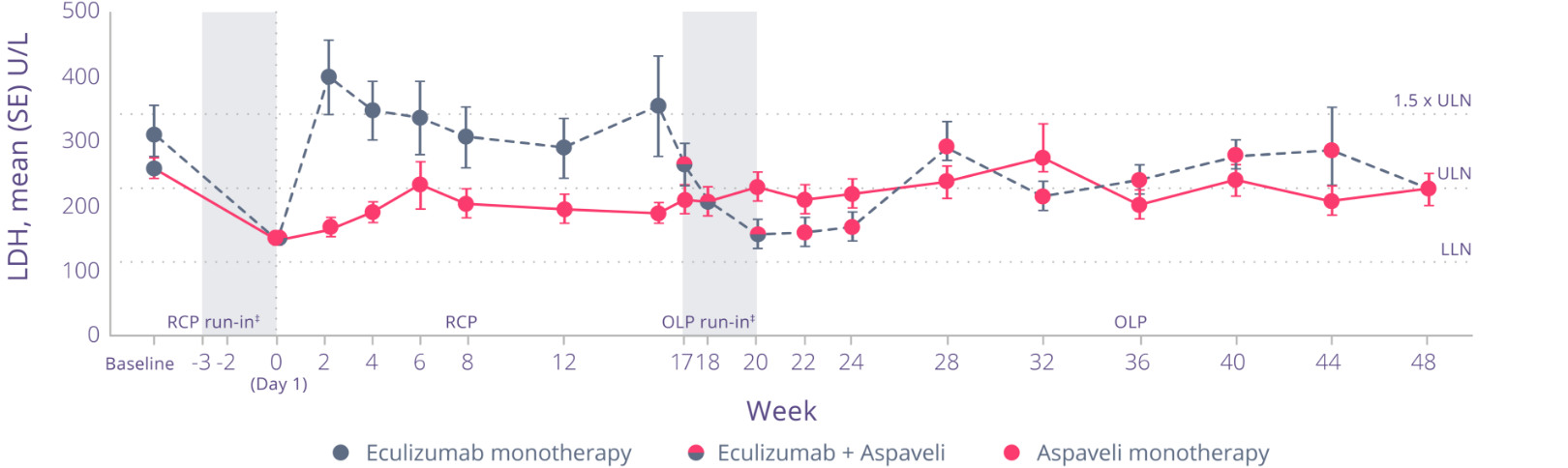
Adapted from Peffault de Latour R et al. Lancet Haematol. 2022.
Through Week 48:§
- Decreases in LDH levels were maintained in patients who continued to receive Aspaveli
- LDH levels decreased in patients who switched from eculizumab to Aspaveli at Week 16
*In adult patients with paroxysmal nocturnal haemoglobinuria (PNH) who have haemolytic anaemia.2
The PRINCE study
The PRINCE study – a phase 3 study in complement inhibitor-naïve adult patients2,5
The PRINCE study was an open-label, randomised, controlled study that enrolled patients with PNH who had not been treated with any complement inhibitor within 3 months prior to enrolment and with haemoglobin levels less than the lower limit of normal (LLN). Eligible patients were randomised in a 2:1 ratio to receive pegcetacoplan or supportive care (e.g., transfusions, corticosteroids, supplements such as iron, folate, and vitamin B12), hereafter referred to as the control arm through the duration of the 26 Week treatment period.
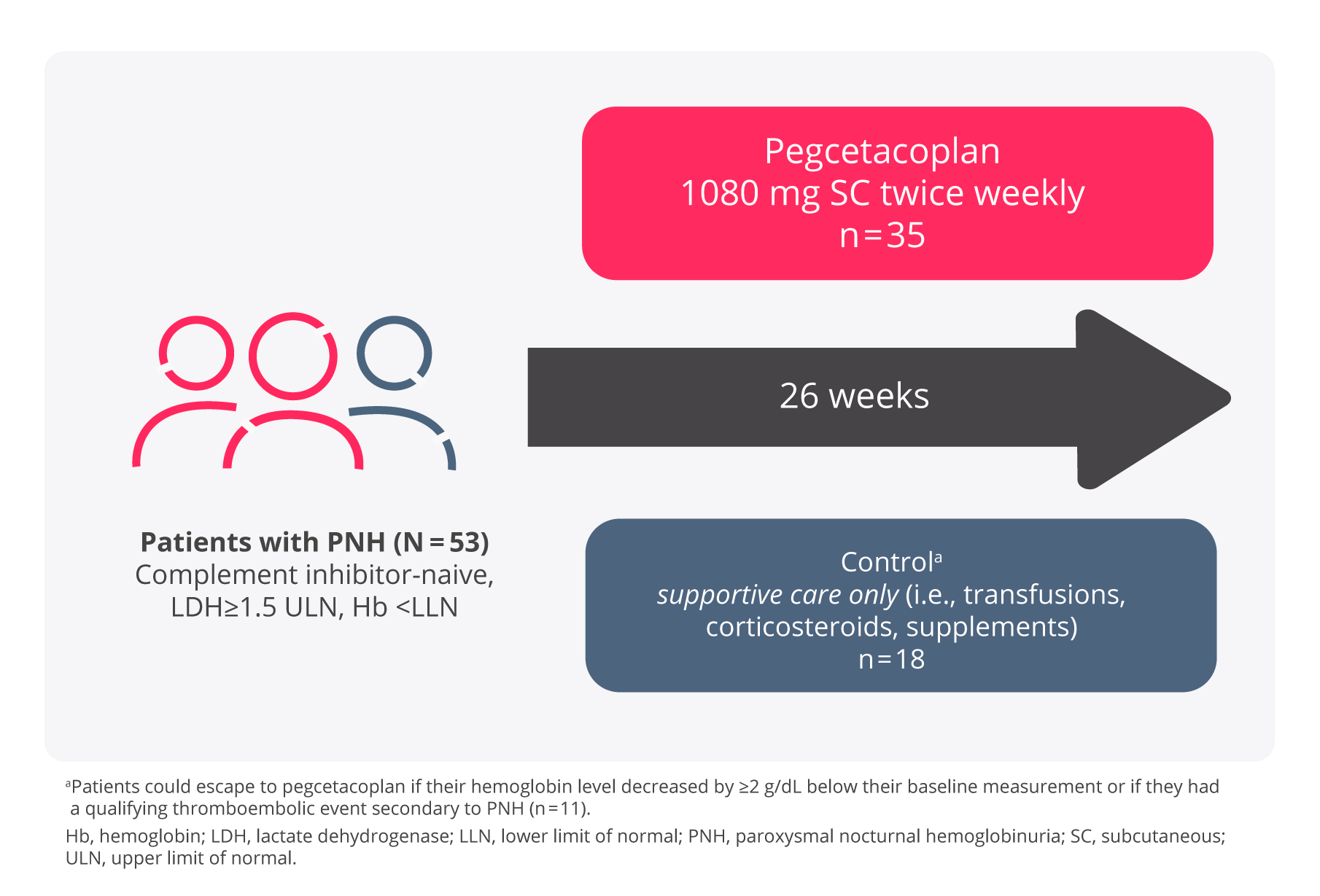
Adapted from Siu Ming Wong, R et al. Blood Adv. 2023.
A total of 53 patients were randomised, 35 to pegcetacoplan and 18 patients to the control arm. Demographics and baseline disease characteristics were generally well balanced between treatment arms. Eleven of 18 patients randomised to the control arm transitioned to pegcetacoplan because their haemoglobin levels decreased by ≥2 g/dL below baseline. Of the 53 randomised patients, 52 (97.8%) received prophylactic antibiotic therapy according to local prescribing guidelines. Overall, 53 patients received pegcetacoplan (n = 35) or control (n = 18).
Coprimary endpoints
The primary and secondary efficacy endpoints were assessed at Week 26.
• Hemoglobin stabilisation (avoidance of >1-g/dL decrease in hemoglobin levels without transfusions) from baseline through Week 26.
• Change in lactate dehydrogenase (LDH) from baseline.
Key secondary end points
Nine secondary end points were tested in a hierarchical manner at Week 26:
1. Hemoglobin response (defined as hemoglobin increase ≥1 g/dL from baseline).
2. CFB in absolute reticulocyte count (ARC).
3. CFB in hemoglobin level.
4. Patients (in percentage) who received transfusion and/or had a >2-g/dL decrease from baseline in hemoglobin level.
5. Transfusion avoidance (defined as no transfusions during the 26-week RCP).
6. Number of PRBC units transfused during the 26-week RCP.
7. CFB in the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue) scale scores.
8. CFB in global health status/QoL scores using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) instrument.
9. ARC normalisation (defined as ARC < ULN [male: from 10 × 109 to 140 × 109 cells per L; and female: from 10 × 109 to 120 × 109 cells per L]).
Three additional secondary end points at Week 26 were included:
1. Patients (%) who had a clinically meaningful improvement in FACIT-Fatigue score (≥3-point increase.
2. Hemoglobin normalisation (defined as hemoglobin levels ≥ LLN [male: ≥13.6 g/dL; and female: ≥12.0 g/dL]).
3. LDH normalization (defined as LDH levels ≤ ULN [226 U/L]).
CFB, change from baseline
Hemoglobin stabilisation
Hemoglobin stabilisation and change in LDH from baseline2,5
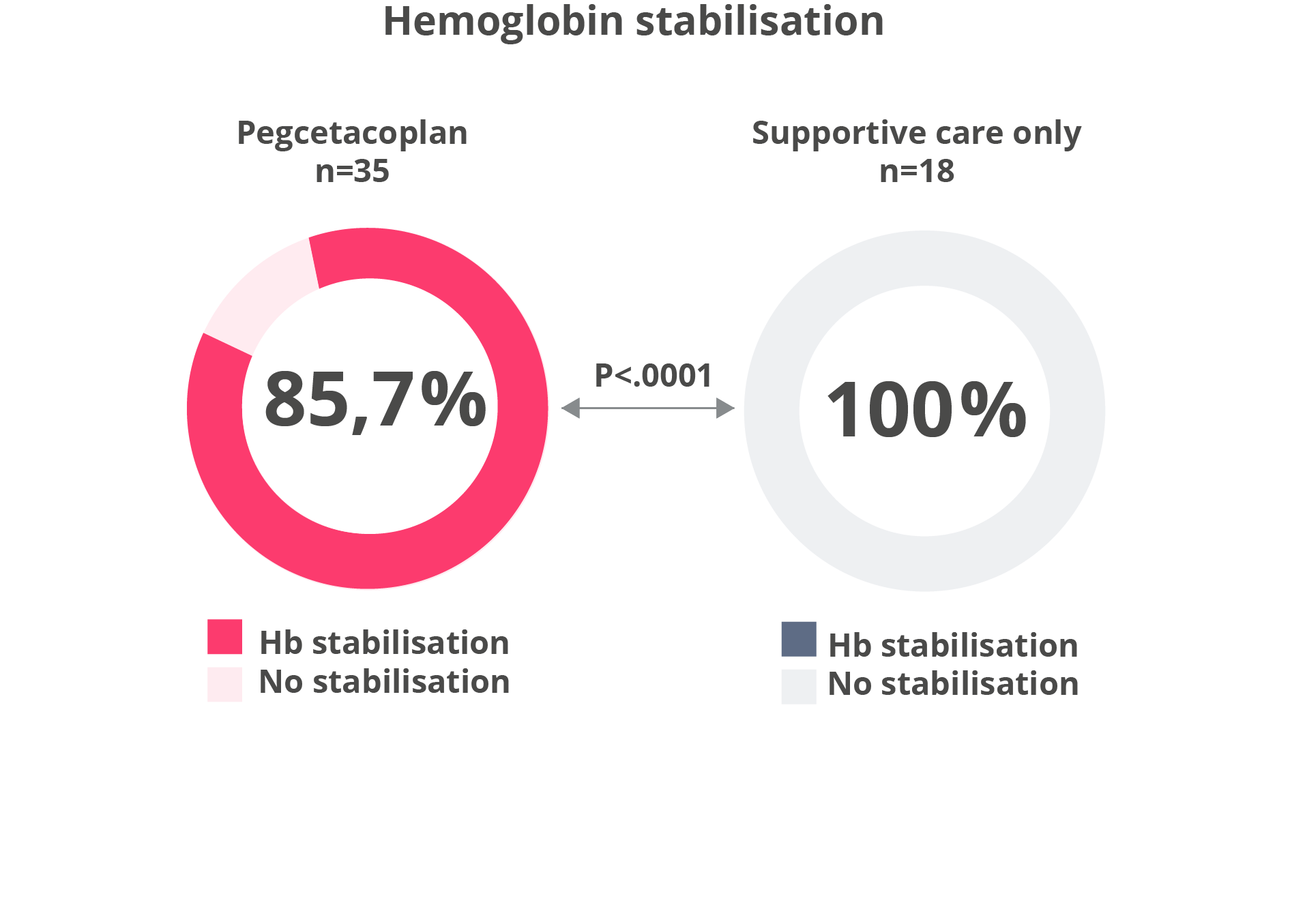
Adapted from Siu Ming Wong, R et al. Blood Adv. 2023 .
Pegcetacoplan was also superior to control for change from baseline in LDH (least square mean change: pegcetacoplan, −1870.5 U/L; control, −400.1 U/L; difference, −1470.4 U/L; 95% CI, −2113.4 to −827.3; P < .0001). The difference between pegcetacoplan and the control arm was -1 470 (95% CI, -2 113 to 827). Treatment differences between the pegcetacoplan and the control arm were evident at Week 2 and were maintained through Week 26. LDH concentrations in the control arm remained elevated.
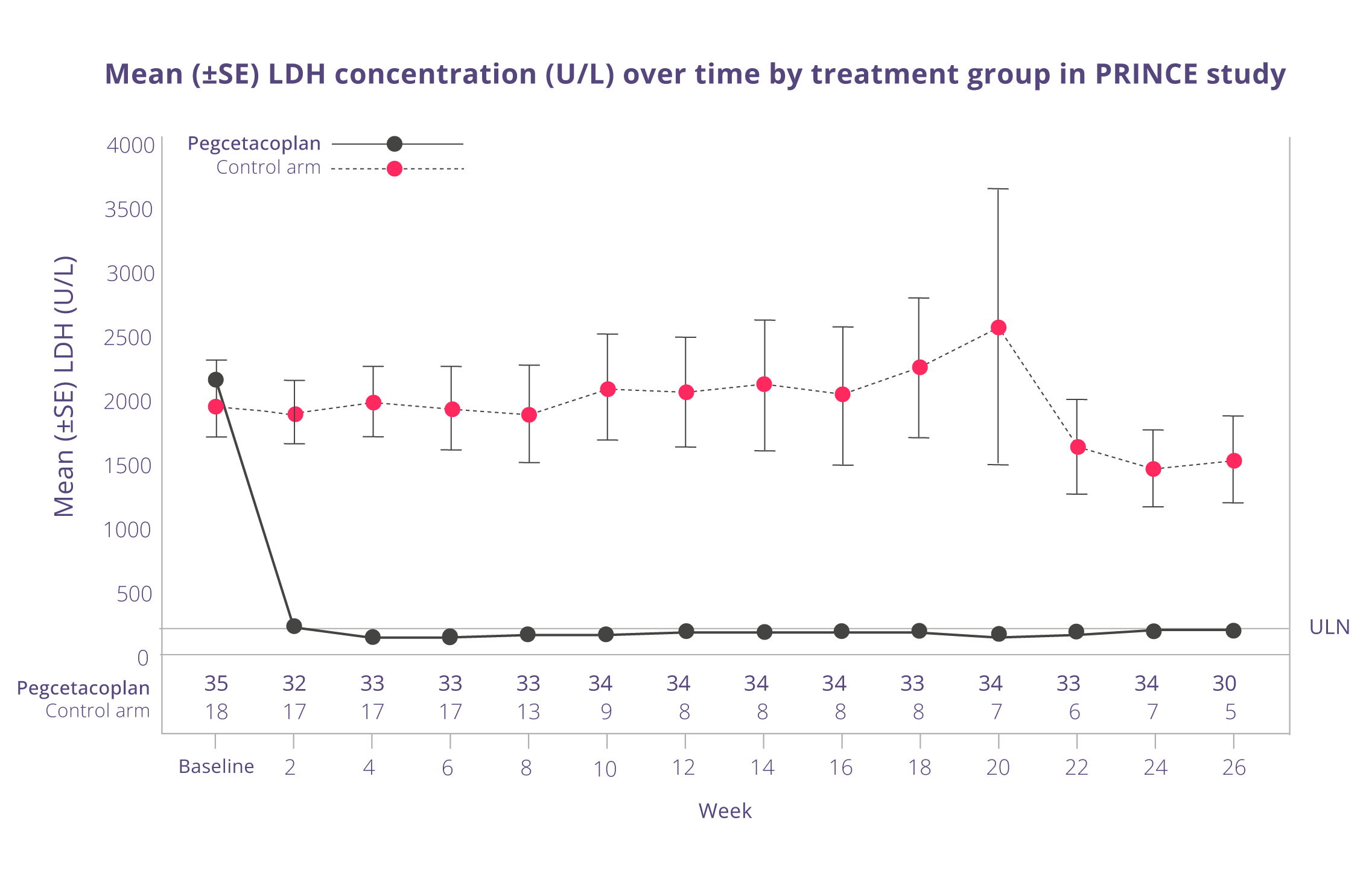
Adapted from Siu Ming Wong, R et al. Blood Adv. 2023.
Key secondary efficacy endpoints
For the selected key secondary efficacy endpoints of haemoglobin response in the absence of transfusions, change in haemoglobin level, and change in ARC, the group treated with pegcetacoplan demonstrated a significant treatment difference versus the control arm.
More than 90% of pegcetacoplan-treated patients were transfusion-free for 26 Weeks (P < .0001) vs <6% in the control group.
Key secondary endpoints analysis in PRINCE study
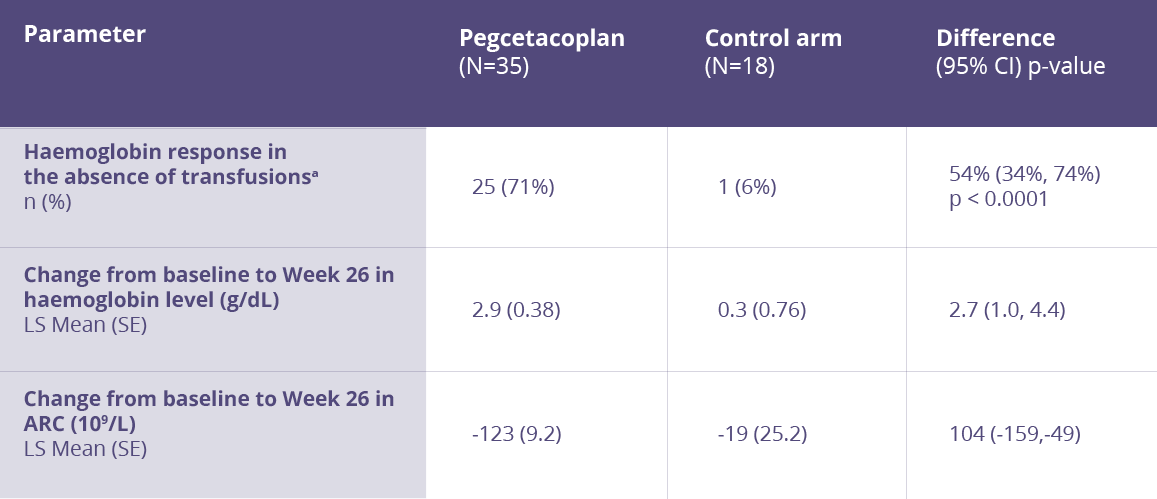
aHaemoglobin response was defined as a ≥1 g/dL increase in haemoglobin from baseline at Week 26.
ARC = Absolute reticulocyte count, CI = Confidence interval, LS = Least square, SE = Standard error
Adapted from Siu Ming Wong, R et al. Blood Adv. 2023.
References
1. Hillmen P et al. N Engl J Med. 2021;384:1028–1037. 2. Aspaveli (pegcetacoplan) Summary of Product Characteristics 08/2024. 3. Peffault de Latour R et al. Lancet Haematol. 2022;9:e648–659. 4. Hillmen P et al. N Engl J Med. 2021; 384(suppl):1–16. 5. Siu Ming Wong R et al. Blood Adv. 2023 Jun 13;7(11):2468-2478.