Dosing
Doptelet is an oral TPO-RA with
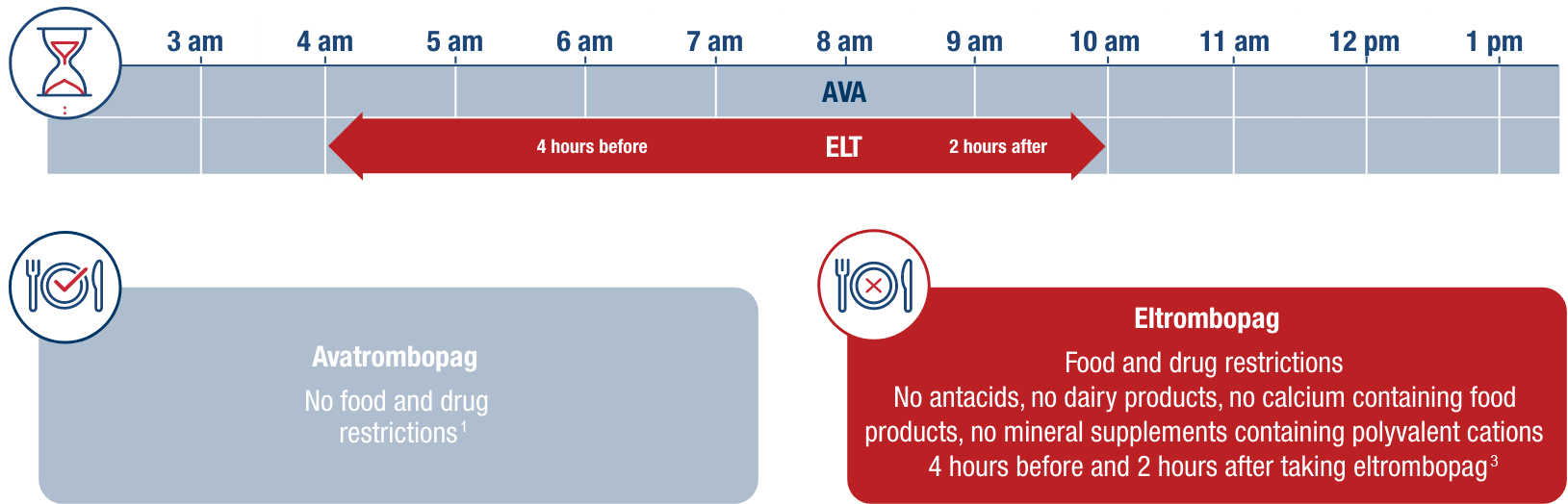
no food-type restrictions1,2
Doptelet can be taken with any type of food1
Example timeline of once-daily administration for avatrombopag vs eltrombopag in adult patients with chronic ITP.1,3 As shown below, patients must adhere to food and drug restrictions before and after taking eltrombopag, or risk a significant 59% decline in eltrombopag absorption.3
Only Doptelet meets patient preference for an oral treatment taken with any type of food1,2,4
As the only TPO-RA with no food-type restrictions, Doptelet offers sustained efficacy in a way that aligns with patient preferences.1,2,4
In the TRAPeze study, adult patients with chronic ITP were:*
7x** more likely to choose oral treatment vs injection4
4x*** more likely to choose oral treatment with no food-type restrictions vs with4
*The TRAPeze study is an ongoing pan-European, cross-sectional, exploratory, observational survey that aims to explore patients’ experiences with TPO-RAs and their preferences towards TPO-RA treatment attributes. Included are results from the UK and Ireland cohort of the TRAPeze study, consisting of 32 people with ITP who had been treated with eltrombopag or romiplostim (the only TPO-RAs available at the time of the study). The survey consisted of two sections on treatment preference and disease burden. The treatment preference section consisted of 10 sets of scenarios, each requiring participants to choose between two hypothetical treatment scenarios that correspond to different TPO-RA attributes. The disease burden section consisted of 101 questions on demographics, disease characteristics, treatment history, overall satisfaction with therapy, impact of ITP on work, productivity, personal life and healthcare resource utilisation.4
**7.4 (95% CI 3.6 - 15.1).
***3.6 (95% CI 1.8 - 6.8).
International guidelines recommend considering patient preference, adherence, and impact on quality of life when it comes to treatment choice.5-6
No need for weekly clinic visits for administration, training, or special storage conditions with Doptelet1
One dosage strength for manageable adjustment1
Begin at 20 mg once daily and titrate up and down as needed, based on your patient’s routine platelet count.1
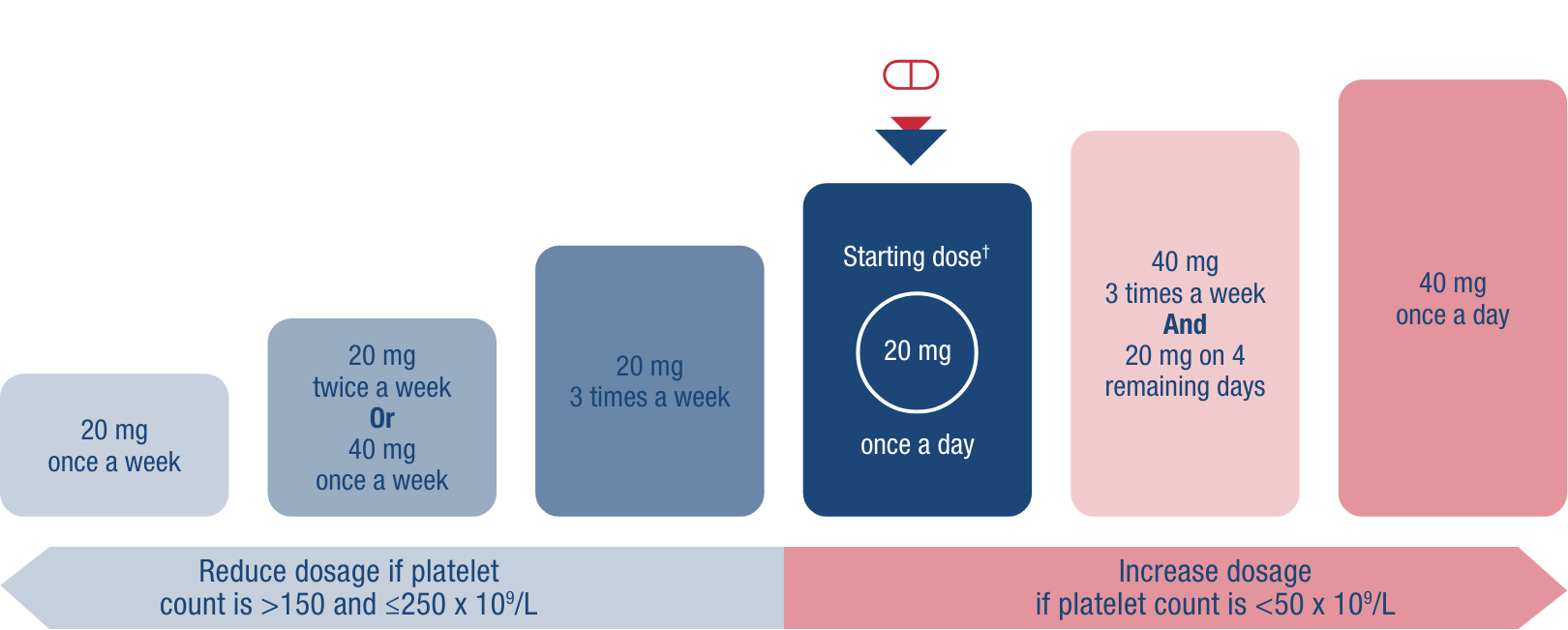
†Initial dose regimen for all patients except those taking moderate or strong dual inducers or moderate or strong dual inhibitors of CYP2C9 and CYP3A4/5, or of CYP2C9 alone.1
Doptelet is indicated for the treatment of severe thrombocytopenia in adult patients with chronic liver disease who are scheduled to undergo an invasive procedure.1
Doptelet is indicated for the treatment of primary chronic immune thrombocytopenia (ITP) in adult patients who are refractory to other treatments (e.g. corticosteroids, immunoglobulins).1
AVA, avatrombopag; ELT, eltrombopag; ITP, immune thrombocytopenia; TPO-RA, thrombopoietin receptor agonist.
References
1. Doptelet Summary of Product Characteristics. 22/05/2025. 2. Jurczak W et al. Br J Haematol. 2018; 183(3):479–490. 3. Revolade Summary of Product Characteristics. 05/2023. 4. McDonald V et al. Hematology. 2021; 26(1): 799–808. 5. Neunert CE et al. Blood Adv. 2019; 3(23):3829–3866. 6. Provan D et al. Blood Adv. 2019; 3(22):3780–3817.


