Guidelines
Doptelet is a guideline-recommended TPO-RA1
ICR, ASH and European JWG recommend limiting steroid use and moving onto TPO-RAs1,2
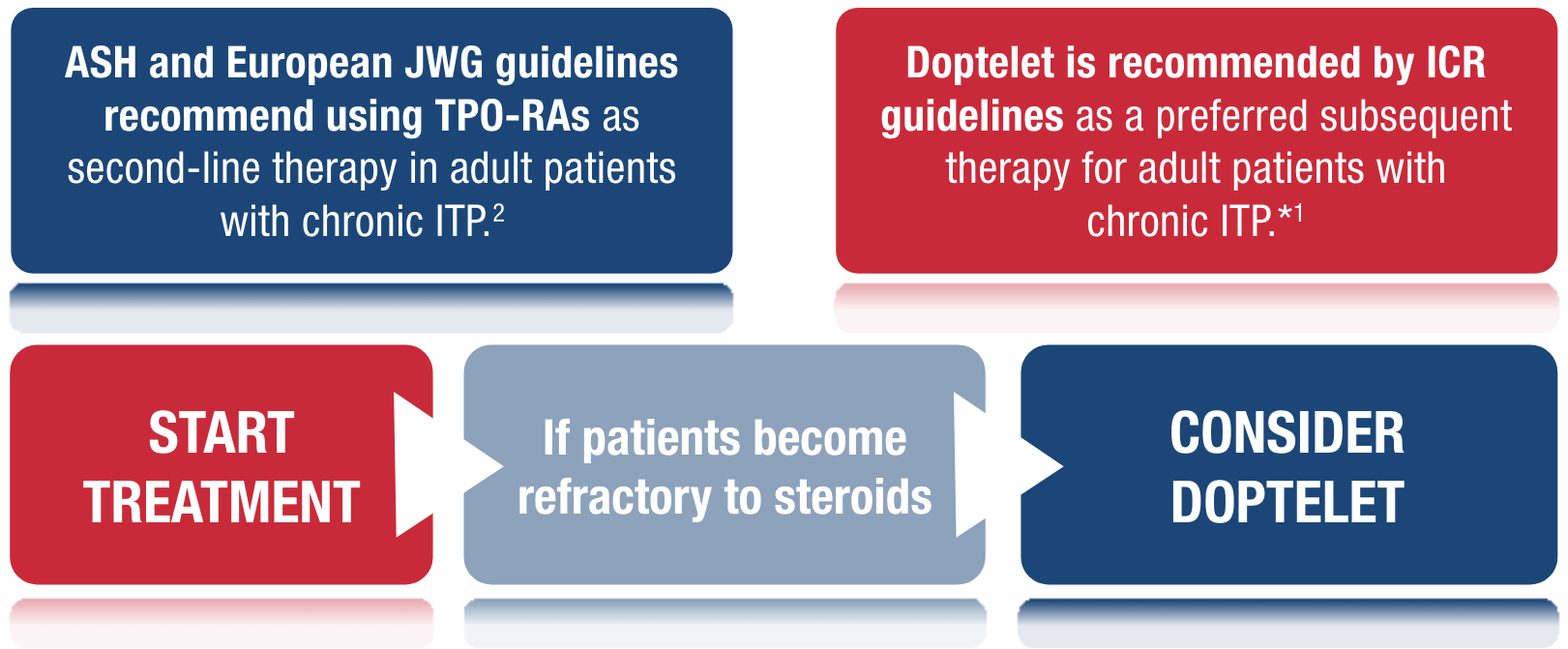
Guidelines encourage shared decision making and recommend considering patient preference,
adherence and impact on quality of life when it comes to treatment choice.1,2
*Grade A recommendation, ≥1 RCT as part of a body of literature of overall good quality and consistency addressing specific recommendation; evidence level Ib, evidence obtained from ≥1 RCT.1
Doptelet is indicated for the treatment of severe thrombocytopenia in adult patients with chronic liver disease who are scheduled to undergo aninvasive procedure. 3
Doptelet is indicated for the treatment of primary chronic immune thrombocytopenia (ITP) in adult patients who are refractory to other treatments (e.g. corticosteroids, immunoglobulins). 3
ASH, American Society of Hematology; ICR, International Consensus Report; ITP, immune thrombocytopenia; JWG, Joint Working Group; RCT, randomised control trial; TPO-RA, thrombopoietin receptor agonist.
References
1. Provan D, Arnold DM, Bussel JB, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3(22):3780-3817. 2. Neunert C, Terrell DR, Arnold DM, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia [published correction appears in Blood Adv. 2020 Jan 28;4(2):252]. Blood Adv. 2019;3(23):3829-3866. 3. Doptelet Summary of Product Characteristics. 22/05/2025


