Half-life extension using Fc fusion technology
Fc fusion technology extends half-life vs SHL FVIII treatment1–3
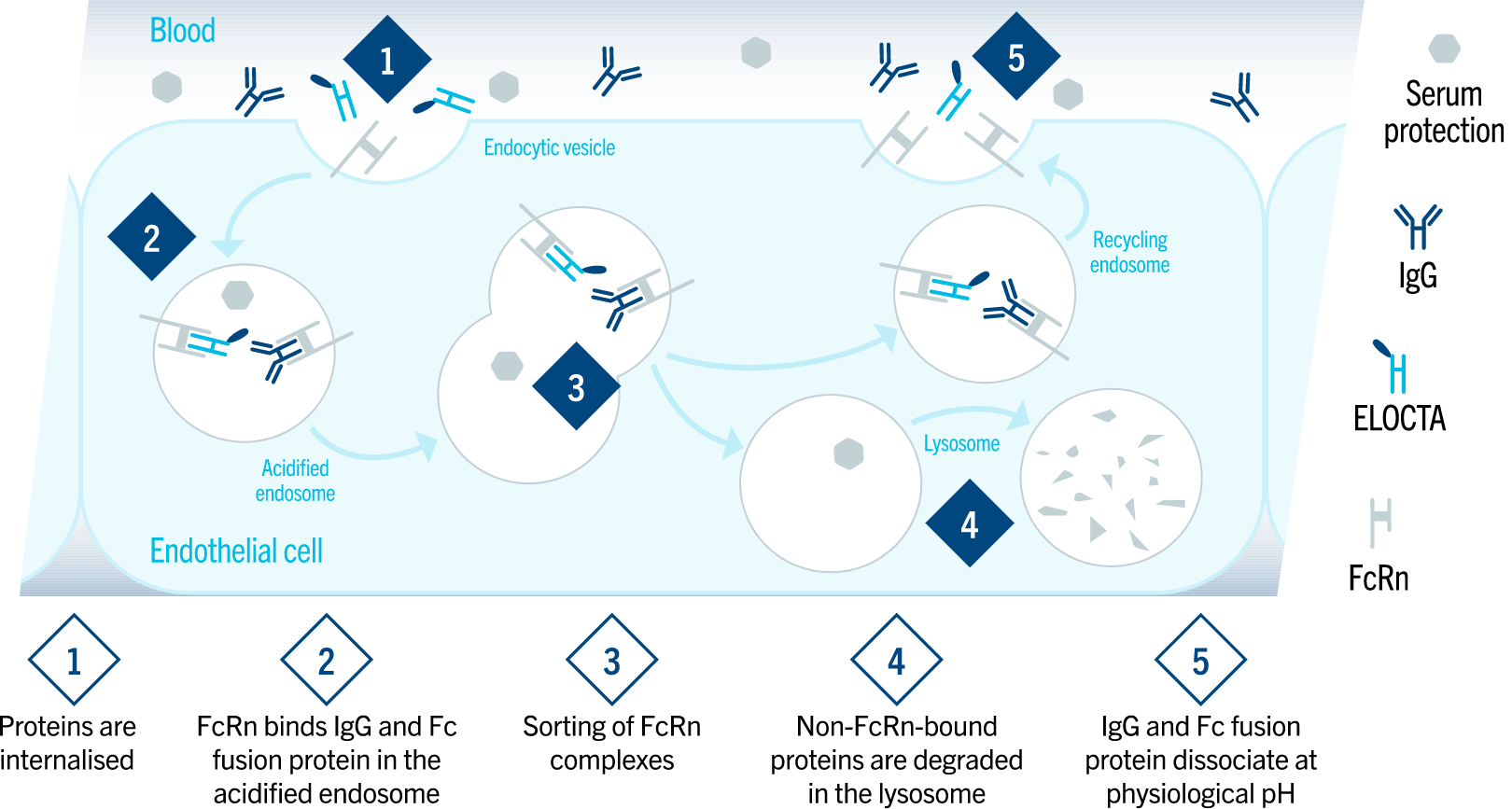
ELOCTA leverages a natural pathway for half-life extension.1
ELOCTA consists of B domain-deleted rFVIII genetically fused to the Fc portion of IgG1.4
The Fc portion of ELOCTA binds to the neonatal Fc receptor, called FcRn. FcRn is part of the naturally occurring IgG recycling pathway which reduces lysosomal degradation of Fc-containing proteins. This reduced clearance results in the prolonged half-life of ELOCTA in human plasma.3–5
ELOCTA leverages a natural recycling pathway to extend half-life1,4,5
ELOCTA is produced using the well-established human HEK293 cell line.3,6
ELOCTA consists of natural components and does not accumulate in the body.3,6,7
Learn more about Fc fusion technology
Find out more about what Fc fusion technology is and how it uses a natural pathway in the body to extend half-life.
ELOCTA uses Fc fusion technology to increase the time spent at higher FVIII levels vs SHL FVIII treatment.
EHL, extended half-life; Fc, fragment crystallisable; FcRn, neonatal Fc receptor; FVIII, factor VIII; IgG, Immunoglobulin G; SHL, standard half-life.
References
1. ELOCTA SmPC 01/2021. 2. Berntorp E, et al. Haemophilia 2016;22(3):389–396 3. Dumont J, et al. Blood 2012;119(13):3024–3030. 4. Shapiro A. Expert Opin Biol Ther 2013;13(9):1287–1297 5. Roopenian DC, Akilesh S. Nat Rev Immunol 2007;7(9):715–725. 6. McCue J, et al. Biologicals 2015;43(4):213–219. 7. Powell JS, et al. Blood 2012;119(13):3031–3037.


